Grading severity of microscopic vascular invasion was independently associated with recurrence and survival following hepatectomy for solitary hepatocellular carcinoma
Highlight box
Key findings
• Grading severity of microscopic vascular invasion (MVI) was independently associated with recurrence-free survival (RFS) and overall survival (OS) following hepatectomy for solitary hepatocellular carcinoma (HCC).
• Enhanced surveillance for recurrence and potentially adjuvant therapy may be considered for patients with MVI, especially individuals with more severe MVI grading (M2).
What is known and what is new?
• On the basis of the three-tiered MVI grading system proposed by the Liver Cancer Pathology Group of China, which combines the distance of MVI (under or over 1 cm) from the main tumor and the number of MVI detected under microscopy, the grading severity of MVI can be divided into three classifications: M0, M1, and M2. Several studies have noted that the presence of MVI is an aggressive biological characteristic of HCC, and MVI may be one of the most crucial risk factors for postoperative recurrence and worse survival following hepatectomy for HCC.
• The present study investigated the association between the grading severity of MVI and RFS and OS among patients with solitary HCC who underwent hepatectomy. Results showed that both M1 and M2 were independent risk factors for RFS and OS, with median RFS rates of 35.1 and 11.6 months, and OS rates of 62.3 and 30.6 months, respectively. These findings suggest that enhanced surveillance for recurrence and potentially adjuvant therapy may be considered for patients with MVI, especially those with more severe MVI grading (M2).
What is the implication, and what should change now?
• Prevention and early identification of HCC recurrence is an important significant strategy to improve long-term oncologic survival among patients undergoing hepatectomy for HCC. The data from the current study suggests that future clinical trials should target assessment of adjuvant therapy among patients with M2 MVI who were at the highest risk of postoperative recurrence and poor survival.
• World-wide efforts to standardize a tissue sampling protocol and a grading system of MVI in HCC are required to decrease the gap in management of HCC among different countries.
Introduction
Hepatocellular carcinoma (HCC) represents the fourth leading cause of cancer-related death worldwide with an estimated mortality of >1 million patients in 2025 (1,2). Endorsed by the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL), the Barcelona Clinic Liver Cancer (BCLC) staging system indicates that solitary HCC without macrovascular invasion and distant metastasis (BCLC stage 0/A), regardless of tumor size, should be considered for curative-intent surgical treatment including hepatectomy and liver transplantation, which may provide a chance of cure (3-5). Unfortunately, long-term survival after hepatectomy for HCC is still compromised by high rates of postoperative recurrence, which can range from 40% to 60% within 5 years after surgery even among patients with solitary HCC (4,6-10). Therefore, a better understanding of clinicopathological characteristics and recurrence-related risk factors for patients with solitary HCC is needed. In particular, even though identified as “early stage” by the BCLC staging system, patients with solitary HCC can still be at a heterogeneous risk of recurrence, and a subset of patients may need more stringent recurrence surveillance and/or effective adjuvant therapy to improve long-term outcomes.
For solitary HCC, many studies have revealed that the most pivotal determinant of postoperative HCC recurrence is the presence of microscopic vascular invasion (MVI), which is widely regarded as a typical characteristic of HCC biological aggressiveness (8,11-17). The incidence of MVI in surgically resected specimens ranges from 15–57% among patients with solitary HCC, while the adjusted increased risk of postoperative recurrence after hepatectomy among patients with solitary HCC has been reported to be as high as 40–60% (4,6-10,18,19). MVI is generally defined as the presence of a cluster of tumor cells in microscopic vessels located in the peritumoral liver (20-22). However, the impact of MVI grading severity (i.e., the location and number of MVI as detected by microscopy in the peritumoral liver) on recurrence and survival after hepatectomy for HCC has not been well-defined (23,24).
In 2015, the Liver Cancer Pathology Group of China (LCPGC) proposed a seven-point baseline sample collection and three-tiered MVI grading system (MVI-TTG), which has been used to standardize and refine histopathological diagnoses of MVI in resected HCC specimens. The MVI-TTG system was based on the location and number of MVI detected on microscopy in the peritumoral liver (25), which divides the grading severity of MVI into three categories: M0, M1, and M2 (Figure 1). This MVI grading system represents an important grading system over the classic binary classification of “absent” versus “present”. The objective of the current study was to evaluate whether MVI grading severity using the MVI-TTG system was associated with recurrence and long-term survival after hepatectomy of solitary HCC. We present this article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-411/rc).

Methods
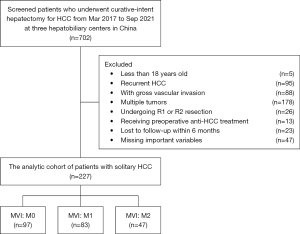
Patients undergoing curative-intent hepatectomy for HCC between March 2017 and September 2021 at three hepatobiliary centers [Department of Hepatobiliary Surgery, Eastern Hepatobiliary Surgery Hospital, Second Military Medical University (Navy Medical University); Department of General Surgery, Cancer Center, Division of Hepatobiliary and Pancreatic Surgery, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou; Department of Hepatobiliary Surgery, Mengchao Hepatobiliary Hospital, Fujian Medical University] in China were identified and included in the analytic cohort. The diagnosis of HCC was confirmed on postoperative histopathological examination. Curative hepatectomy was defined as complete macro- and microscopic removal of tumor(s) (R0 resection). Exclusion criteria included patients with: (I) less than 18 years old of age; (II) recurrent HCC; (III) gross vascular invasion; (IV) multiple tumors (more than 2 nodules); (V) R1 or R2 resection; (VI) preoperative anti-HCC treatment; and (VII) lost to follow-up within 6 months after surgery or with missing potentially important prognostic variables, such as preoperative AFP level, tumor differentiation and resection margin in very few patients. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional review board approval was obtained from Eastern Hepatobiliary Surgery Hospital of Shanghai (No. EHBHKY2021-K-035); informed consent for the usage of data for research from all the enrolled HCC patients was obtained on hospital admission.
Data collection
Clinical characteristics and operative variables relative to the patient, liver, tumor, and operation were collected. Patient-related factors included sex, age, obesity (body mass index >25 kg/m2), diabetes mellitus, American Society of Anesthesiologists (ASA) score. Liver-related factors included hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, cirrhosis, portal hypertension, Child-Pugh grade. Tumor-related factors included preoperative alpha-fetoprotein (AFP) level, tumor size, satellite nodules, tumor encapsulation and tumor differentiation. Operation-related factors included intraoperative blood loss, intraoperative blood transfusion, type of resection (anatomical or non-anatomical), extent of hepatectomy (minor or major), and resection margin (<1.0 or ≥1.0 cm). Portal hypertension was defined as the presence of splenomegaly with esophageal varicosities. Minor hepatectomy was defined as removal of fewer than 3 Couinaud segments, while major hepatectomy was defined as removal of 3 or more segments. Anatomical resection was identified by the Brisbane 2000 system, while non-anatomical resection incorporated limited or wedge resections. In previously published studies, identification of number of HCC is based on preoperative radiological and clinical features, while satellite nodules and MVI can only be detected histopathlogically in resected liver specimens (25,26). We have published one correspondence on the difference between satellite nodules and MVI (27).
Grading severity of MVI
The 7-point baseline sampling protocol was performed on all the HCC specimens based on the “Evidence-based Practice Guidelines for Standardized Pathological Diagnosis of Primary Liver Cancer in China” (25). Four tissue specimens were sampled at 3, 6, 9, and 12 o’clock positions at the junction of the tumor and the adjacent liver tissues in a 1:1 ratio, together with one specimen sampled at the intra-tumoral zone and two specimens sampled within 1 cm from the tumor capsule and over 1 cm from the tumor capsule or tumor margin. The grading severity of MVI was identified by two senior pathologists based on the Chinese MVI-TTG system (25). MVI was defined as presence of a cluster of HCC cells within a vascular lumen space of liver tissue lined by endothelial cells, mainly seen in portal vessels branches detected on microscopy. Grading severity of MVI was graded as: M0 (no MVI), M1 (1–5 sites of MVI appearing in the tumor-adjacent liver tissue ≤1.0 cm away from the main tumor), M2 (>5 sites of MVI appearing in the tumor-adjacent liver tissue ≤1.0 cm, and/or any MVI occurring in distant liver tissue >1.0 cm away from the main tumor).
Follow-up
After discharge, patients were followed-up in accordance with a standardized surveillance protocol to assess for HCC recurrence. Specifically, surveillance was performed once every 2 or 3 months for the first 2 years and then every 6 months afterwards. At each follow-up appointment, patients were screened with serum AFP level, as well as abdominal ultrasound imaging, contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) for surveillance of recurrence. When recurrence was suspected, patients underwent further examination such as positron emission tomography or bone scanning as clinically indicated. A diagnosis of tumor recurrence was based on the typical findings of dynamic MRI or CT, with or without elevation of serum AFP levels. Patients with confirmed recurrent HCC received further management under the evaluation by a multidisciplinary team that included the treating surgeon.
Study endpoints
The study endpoints were recurrence-free survival (RFS) and overall survival (OS). RFS was calculated from the date of operation to the date of initial diagnosis of HCC recurrence, or the date of death or the last follow-up; OS was calculated from the date of operation to either the date of death or the date of the last follow-up.
Statistical analysis
Data were reported as mean ± standard deviation or frequency and percentage, as appropriate. Differences between groups were analyzed by the χ2 or Fisher exact probability test for categorical variables, and the student t-test or Mann-Whitney U test for continuous variables. RFS and OS were calculated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariate Cox proportional hazard regression analyses were used to identify risk factors contributing to RFS and OS following hepatectomy for solitary HCC. Variables with a P value of <0.10 on univariate analysis were subjected to multivariate Cox-regression model using a forward stepwise variable selection. All statistical tests were two-tailed and a value of P<0.05 was considered statistically significant. All analyses were performed using statistical software (SPSS 26.0, Inc., Chicago, IL, USA).
Results
Overall, 227 patients who underwent curative hepatectomy for solitary HCC (BCLC stage 0/A) were included in the analytic cohort (Figure 2); 203 (89.4%) were male and 24 (10.6%) were female with a median age of 50 years. Postoperative microscopic examination of the resected surgical specimens demonstrated MVI grading severity M0, M1, and M2 in 97 (42.7%), 83 (36.6%), and 47 (20.7%) patients, respectively.
Comparisons of clinical characteristics and operative variables
Clinical characteristics and operative variables of three different MVI groups are noted in Table 1. There were differences noted among the three groups mainly related to tumor- and operation-related variables, including preoperative AFP level, tumor size, satellite nodules, tumor encapsulation, tumor differentiation, intraoperative blood transfusion, and extent of hepatectomy (all P<0.05).
Table 1
| Variables | M0 (n=97) | M1 (n=83) | M2 (n=47) | P value |
|---|---|---|---|---|
| Male sex | 86 (88.7) | 75 (90.4) | 42 (89.4) | 0.934 |
| Age*, years | 53±11 | 50±12 | 49±10 | 0.125 |
| Obesity (body mass index >25 kg/m2) | 24 (24.7) | 24 (28.9) | 14 (29.8) | 0.750 |
| Diabetes mellitus | 5 (5.2) | 9 (10.8) | 2 (4.3) | 0.233 |
| ASA score >2 | 14 (14.4) | 15 (18.1) | 5 (10.6) | 0.511 |
| HBV (+) | 85 (87.6) | 75 (90.4) | 42 (89.4) | 0.840 |
| HCV (+) | 2 (2.1) | 1 (1.2) | 3 (6.4) | 0.187 |
| Cirrhosis | 77 (79.4) | 58 (69.9) | 33 (70.2) | 0.280 |
| Portal hypertension | 22 (22.7) | 23 (27.7) | 7 (14.9) | 0.247 |
| Child-Pugh grade | ||||
| A | 81 (83.5) | 76 (91.6) | 40 (85.1) | 0.262 |
| B | 16 (16.5) | 7 (8.4) | 7 (14.9) | |
| Preoperative AFP level >400 μg/L | 23 (23.7) | 36 (43.4) | 25 (53.2) | 0.001 |
| Tumor size*, cm | 5.2±3.1 | 7.3±4.1 | 8.4±4.6 | <0.001 |
| Satellite nodules | 11 (11.3) | 18 (21.7) | 14 (29.8) | 0.022 |
| Incomplete tumor encapsulation | 28 (28.9) | 60 (72.3) | 30 (63.8) | <0.001 |
| Poorly tumor differentiation | 38 (39.2) | 60 (72.3) | 37 (78.7) | <0.001 |
| Intraoperative blood loss >600 mL | 22 (22.7) | 17 (20.5) | 18 (38.3) | 0.061 |
| Intraoperative blood transfusion | 21 (21.6) | 17 (20.5) | 20 (42.6) | 0.011 |
| Anatomical resection | 30 (30.9) | 37 (44.6) | 17 (36.2) | 0.166 |
| Major hepatectomy | 11 (11.3) | 23 (27.7) | 19 (40.4) | <0.001 |
| Resection margin <1.0 cm | 39 (40.2) | 43 (51.8) | 25 (53.2) | 0.193 |
Values in parentheses are percentages unless indicated otherwise. *, values are mean ± standard deviation. ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein.
Comparisons of long-term oncologic outcomes
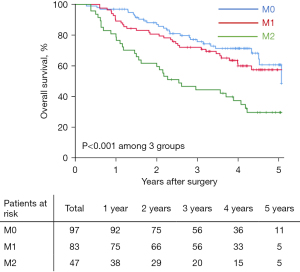
With a median follow-up of 42.7 (range, 2.6 to 70.8) months, 93 (41.0%) patients had died and 131 (57.7%) developed HCC recurrence. Table 2 demonstrates long-term oncologic outcomes among the three different MVI grading patient groups. In particular, overall recurrence rates among patients in the M0, M1, and M2 groups were 46.4% (45/97), 57.8% (48/83), and 80.9% (38/47), respectively (P<0.001). Overall, 5-year mortality rates among these three groups were 28.9% (28/97), 39.8% (33/83), and 68.1% (32/47), respectively (P<0.001). Median RFS and OS among patients with M0, M1, and M2 were 38.3 and 66.8 months, 35.1 and 62.3 months, and 11.6 and 30.6 months respectively (all P<0.001). 5-year RFS rates among patients in the M0, M1, and M2 groups was 44.4%, 36.5%, and 17.5%, respectively, while 5-year OS among patients in the M0, M1, and M2 groups were 60.7%, 57.4%, and 29.7%, respectively (Table 2). Compared with the M0 group, patients in both the M1 and M2 groups had decreased RFS [hazard rate (HR) 1.29, 95% confidence interval (CI): 1.00 to 1.93, P=0.058; and HR 2.51, 95% CI: 1.62 to 3.86, P<0.001, respectively) (Figure 3), as well as worse OS after hepatectomy for solitary HCC (HR 1.22, 95% CI: 0.90 to 1.86, P=0.089; and HR 2.85, 95% CI: 1.71 to 4.73, P<0.001, respectively) (Figure 4).
Table 2
| Variables | M0 (n=97) | M1 (n=83) | M2 (n=47) | P value |
|---|---|---|---|---|
| Death during follow-up | 28 (28.9) | 33 (39.8) | 32 (68.1) | <0.001 |
| Recurrence during follow-up | 45 (46.4) | 48 (57.8) | 38 (80.9) | <0.001 |
| Pattern of initial recurrence | ||||
| Intrahepatic only | 32 (33.0) | 33 (39.7) | 20 (42.5) | 0.464 |
| Extrahepatic only | 6 (6.2) | 5 (6.0) | 4 (8.5) | 0.840 |
| Intra- & extrahepatic | 7 (7.2) | 10 (12.0) | 10 (21.3) | 0.050 |
| RFS, months* | 38.3 (27.8, 48.8) | 35.1 (18.2, 52.0) | 11.6 (6.2, 17.0) | <0.001 |
| 1-year rate (%) | 89.6 | 73.5 | 48.9 | |
| 3-year rate (%) | 54.6 | 48.8 | 29.5 | |
| 5-year rate (%) | 44.4 | 36.5 | 17.5 | |
| OS, months* | 66.8 (NA) | 62.3 (43.7, 82.9) | 30.6 (17.9, 43.3) | <0.001 |
| 1-year rate (%) | 96.9 | 89.2 | 80.9 | |
| 3-year rate (%) | 75.8 | 72.1 | 44.4 | |
| 5-year rate (%) | 60.7 | 57.4 | 29.7 |
Values in parentheses are percentages unless indicated otherwise. *, values are median (95% confidence intervals). NA, not attained; OS, overall survival; RFS, recurrence-free survival.

Univariate and multivariate analyses of RFS and OS
Univariate and multivariate Cox-regression analyses of RFS and OS after hepatectomy for solitary HCC are noted in Tables 3,4. On multivariate analysis, compared with M0 disease, M1 and M2 MVI were independently associated with worse RFS (HR 1.20, 95% CI: 1.03 to 1.89, P=0.040; and HR 1.67, 95% CI: 1.06 to 2.64, P=0.027, respectively) and worse OS (HR 1.28, 95% CI: 1.05 to 2.07, P=0.035; and HR 1.97, 95% CI: 1.15 to 3.38, P=0.013) after hepatectomy for solitary HCC.
Table 3
| Variables | Comparisons | UV | MV | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Sex | Male vs. female | 0.98 (0.56–1.71) | 0.949 | |||
| Age | >60 vs. ≤60 years | 0.78 (0.50–1.22) | 0.280 | |||
| ASA score | >2 vs. ≤2 | 0.92 (0.56–1.52) | 0.753 | |||
| Obesity | Yes vs. no | 1.14 (0.78–1.66) | 0.510 | |||
| Diabetes mellitus | Yes vs. no | 1.14 (0.60–2.17) | 0.700 | |||
| HBV (+) | Yes vs. no | 0.77 (0.47–1.26) | 0.295 | |||
| HCV (+) | Yes vs. no | 1.77 (0.72–4.34) | 0.209 | |||
| Cirrhosis | Yes vs. no | 1.01 (0.68–1.49) | 0.964 | |||
| Portal hypertension | Yes vs. no | 0.83 (0.41–1.09) | 0.150 | |||
| Child-Pugh grade | A vs. B | 1.15 (0.70–1.90) | 0.570 | |||
| Preoperative AFP level | >400 vs. ≤400 μg/L | 1.48 (1.04–2.10) | 0.027 | 1.61 (1.12–2.31) | 0.009 | |
| Tumor size | >5.0 vs. ≤5.0 cm | 3.48 (2.33–5.21) | <0.001 | 2.31 (1.49–3.58) | <0.001 | |
| Microscopic invasion grade | M1 vs. M0 | 1.29 (1.00–1.93) | 0.058 | 1.20 (1.03–1.89) | 0.040 | |
| M2 vs. M0 | 2.51 (1.62–3.86) | <0.001 | 1.67 (1.06–2.64) | 0.027 | ||
| Satellite nodules | Yes vs. no | 1.89 (1.28–2.80) | 0.001 | 1.59 (1.06–2.39) | 0.024 | |
| Incomplete tumor encapsulation | Yes vs. no | 1.72 (1.21–2.44) | 0.002 | NS | 0.107 | |
| Poorly tumor differentiation | Yes vs. no | 0.89 (0.63–1.26) | 0.507 | |||
| Intraoperative blood loss | >600 vs. ≤600 mL | 2.19 (1.52–3.17) | <0.001 | NS | 0.557 | |
| Intraoperative blood transfusion | Yes vs. no | 2.33 (1.62–3.35) | <0.001 | 1.77 (1.20–2.60) | 0.004 | |
| Extent of hepatectomy | Major vs. minor | 2.28 (1.58–3.30) | <0.001 | NS | 0.493 | |
| Type of resection | Anatomical vs. non-anatomical |
1.04 (0.73–1.49) | 0.830 | |||
| Resection margin | <1.0 vs. ≥1.0 cm | 2.44 (1.72–3.46) | <0.001 | 1.92 (1.32–2.78) | 0.001 | |
UV, univariate; HR, hazard rate; CI, confidence interval; MV, multivariate; ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; NS, not significant.
Table 4
| Variables | Comparisons | UV | MV | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Sex | Male vs. female | 1.27 (0.62–2.62) | 0.519 | |||
| Age | >60 vs. ≤60 years | 0.66 (0.38–1.14) | 0.134 | |||
| ASA score | >2 vs. ≤2 | 1.21 (0.71–2.08) | 0.498 | |||
| Obesity | Yes vs. no | 1.45 (0.94–2.22) | 0.090 | NS | 0.300 | |
| Diabetes mellitus | Yes vs. no | 1.40 (0.70–2.79) | 0.338 | |||
| HBV (+) | Yes vs. no | 1.32 (0.66–2.63) | 0.430 | |||
| HCV (+) | Yes vs. no | 1.23 (0.39–3.90) | 0.722 | |||
| Cirrhosis | Yes vs. no | 1.18 (0.73–1.91) | 0.494 | |||
| Portal hypertension | Yes vs. no | 1.30 (0.82–2.04) | 0.262 | |||
| Child-Pugh grade | A vs. B | 1.61 (0.96–2.70) | 0.071 | 1.87 (1.09–3.22) | 0.023 | |
| Preoperative AFP level | >400 vs. ≤400 μg/L | 1.82 (1.21–2.74) | 0.004 | 1.96 (1.29–2.99) | 0.002 | |
| Tumor size | >5.0 vs. ≤5.0 cm | 3.53 (2.13–5.85) | <0.001 | 2.73 (1.59–4.70) | <0.001 | |
| Microscopic invasion grade | M1 vs. M0 | 1.22 (0.90–1.86) | 0.089 | 1.28 (1.05–2.07) | 0.035 | |
| M2 vs. M0 | 2.85 (1.71–4.73) | <0.001 | 1.97 (1.15–3.38) | 0.013 | ||
| Satellite nodules | Yes vs. no | 2.86 (1.70–4.80) | <0.001 | NS | 0.191 | |
| Incomplete tumor encapsulation | Yes vs. no | 1.95 (1.27–2.98) | 0.002 | NS | 0.054 | |
| Poorly tumor differentiation | Yes vs. no | 0.81 (0.53–1.22) | 0.306 | |||
| Intraoperative blood loss | >600 vs. ≤600 mL | 2.13 (1.39–3.26) | 0.001 | NS | 0.362 | |
| Intraoperative blood transfusion | Yes vs. no | 2.42 (2.59–3.69) | <0.001 | NS | 0.082 | |
| Extent of hepatectomy | Major vs. minor | 2.54 (1.66–3.88) | <0.001 | NS | 0.617 | |
| Type of resection | Anatomical vs. non-anatomical |
1.34 (0.88–2.02) | 0.171 | NS | 0.215 | |
| Resection margin | <1.0 vs. ≥1.0 cm | 3.06 (1.98–4.71) | <0.001 | 2.67 (1.70–4.19) | <0.001 | |
UV, univariate; HR, hazard rate; CI, confidence interval; MV, multivariate; ASA, American Society of Anesthesiologists; HBV, hepatitis B virus; HCV, hepatitis C virus; AFP, α-fetoprotein; NS, not significant.
In addition to MVI grading severity (M1 and M2), other independent risk factors associated with RFS included preoperative AFP >400 µg/L (HR 1.61, 95% CI: 1.12 to 2.31, P=0.009), tumor size >5.0 cm (HR 2.31, 95% CI: 1.49 to 3.58, P<0.001), satellite nodules (HR 1.59, 95% CI: 1.06 to 2.39, P=0.024), intraoperative blood transfusion (HR 1.77, 95% CI: 1.20 to 2.60, P=0.004) and resection margin <1 cm (HR 1.92, 95% CI: 1.32 to 2.78, P=0.001) (Table 3). Other independent risk factors associated with OS included Child-Pugh grade (HR 1.87, 95% CI: 1.09 to 3.22, P=0.023), preoperative AFP >400 µg/L (HR 1.96, 95% CI: 1.29 to 2.99, P=0.002), tumor size >5.0 cm (HR 2.73, 95% CI, 1.59 to 4.70, P<0.001), and resection margin <1 cm (HR 2.67, 95% CI, 1.70 to 4.19, P<0.001) (Table 4).
Discussion
Several studies have noted that the presence of MVI is an aggressive biological characteristic of HCC, and MVI may be one of the most crucial risk factors for postoperative recurrence and worse survival following hepatectomy for HCC (28,29). On the basis of the MVI-TTG system proposed by the LCPGC, which combines the distance of MVI (under or over 1 cm) from the main tumor and the number of MVI detected under microscopy, the grading severity of MVI can be divided into three classifications: M0, M1, and M2 (25,30). This grading system is a balance which is based on the extensive experience related to the clinical management of HCC patients in China, technical practicality and convenience for pathologists to produce a standardized report for MVI in clinical practice, with a pathological report that can facilitate clinicians to understand better the significance of MVI grading severity, so that clinicians can transmit relevant information to patients with HCC (31). The present study was important because we demonstrated that not only the presence of MVI, but also the MVI grading severity (i.e., the location and number of MVI detected by microscopy) were strongly associated with an increased risk of postoperative recurrence following hepatectomy for solitary HCC. Specifically, more severe MVI grading (M2) was directly correlated with higher recurrence and mortality. In turn, the data strongly suggest that MVI grading severity—but not simply the absence/presence of MVI—had important clinical implications. In particular, information on MVI grading may have implications for postoperative recurrence surveillance and anti-recurrence strategies in patients with solitary HCC.
The MVI-TTG system, which incorporates the location and number of MVI, has previously been shown to associated with postoperative recurrence and survival (21,32). Compared with various other MVI grading systems that focus only on MVI numbers (23) and number of MVI cells (33), the MVI-TTG system balances the value in clinical practice and the technical practicality of pathologist by stratifying HCC patients into different risk groups relative to postoperative recurrence and survival without adding excessive time, cost or pathologist workload. Since its proposal in 2015, the MVI-TTG system has been promoted and used at many major centers in China and has increased the detection of MVI nearly by 10% (30). In the current study, the prognostic role of the MVI-TTG system for patients with solitary HCC was specially studied. The results demonstrated that patients with solitary HCC could be successfully stratified into different risks of postoperative recurrence and survival based on the MVI-TTG system.
As shown in Table 1, there were close relationships between severity of MVI with some tumor characteristics which would also have influenced on the long-term outcomes of patients with HCC after hepatectomy, such as tumor size, satellite nodules, tumor differentiation, and preoperative AFP levels. Multivariate Cox-regression analyses were then performed to adjust the influence of confounding factors. The results identified severity of MVI (both M1 and M2), preoperative AFP level, tumor size, and satellite nodules to be independent risk factors of OS and RFS after hepatectomy for solitary HCC.
As shown in Figures 3,4, although there was a trend in the difference of OS and RFS between patients with M0 and M1, the P value by log-rank test was more than 0.05 by univariate analysis (P=0.089 and 0.053, respectively). Apart from the reason of insufficient sample size, another possible reason is that there was some imbalances in the baseline characteristics between the two groups. For example, compared to patients with M1, patients with M0 who were included in the analysis had a higher proportion of cirrhosis (79.4% vs. 69.9%), and a higher proportion of Child-Pugh grade B (16.5% vs. 8.4%). Multivariate cox-regression analyses were then performed to adjust the influence of confounding factors. As shown in Tables 3,4, significant differences in OS and RFS were achieved not only between patients with M0 and M2, but also between patients with M0 and M1. Therefore, the results of the multivariate analyses confirmed that not only presence or absence of MVI, but also grading severity of MVI are of practical clinical value in evaluating long-term prognosis for patients undergoing curative liver resection for HCC.
Prevention and early identification of HCC recurrence is an important significant strategy to improve long-term oncologic survival among patients undergoing hepatectomy for HCC. However, few adjuvant therapies have previously been demonstrated to have a therapeutic benefit. Although several treatments such as transarterial chemoembolization (TACE), systemic therapy with chemotherapy and kinase inhibitors, have been identified in both the adjuvant and neoadjuvant settings, none have been universally accepted or recommended by current HCC international guidelines (34-36). The STORM trial evaluated the efficacy of sorafenib as adjuvant treatment for HCC patients after hepatectomy or local ablation, and noted no difference in RFS between the control and the treatment groups (37). One explanation for the failure of this study to identify a benefit to sorafenib as an adjuvant therapy was the inclusion of patients with different risks of recurrence in the study cohort. In particular, only patients at high risk of postoperative recurrence may benefit from adjuvant therapy. In turn, the data from the current study suggests that future clinical trials should target assessment of adjuvant therapy among patients with M2 MVI who were at the highest risk of postoperative recurrence and poor survival. Numerous studies have been reported on development of preoperative prediction models for MVI. As patients with M2 have much poorer oncologic prognosis than patients with M0 and M1, it would be clinically useful if M2 tumors could accurately be predicted preoperatively.
The present study had several limitations. Similar to other retrospective studies, there was likely some inherent selection biases. The study was also based solely on data from China; therefore, a majority of patients had HBV-related HCC. Considering that previous studies revealed a potential association between HBV and MVI (38,39), external validation will be needed to validate the study results among HCC patients from Western centers, as well as patients who have HCV infection, non-alcoholic, or alcoholic steatohepatitis. In addition, the absence of presentation of data on inter-observer scoring of microscopic slides, especially for a multicenter study, is another limitation worth mentioning. As such, the MVI-TTG system as proposed by the LCPGC needs further external validation in the future. Nowadays, there are several tissue sampling protocols and subclassifications of MVI in HCC used in the world. World-wide efforts to standardize a tissue sampling protocol and a grading system of MVI in HCC are required to decrease the gap in management of HCC among different countries.
Conclusions
In conclusion, M1 and M2 MVI grade severity was both independently associated with worse RFS and OS after hepatectomy for solitary HCC. MVI grading based on the MVI-TTG system as proposed by the LCPGC can stratify patients into prognostic groups with different risks of postoperative recurrence and survival. Enhanced surveillance for recurrence and potentially effective adjuvant therapy are worth considering among patients with MVI, especially individuals with more severe MVI grading (M2).
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-411/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-411/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-411/coif). T.M.P. serves as an unpaid editorial board member of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Institutional review board approval was obtained from Eastern Hepatobiliary Surgery Hospital of Shanghai (No. EHBHKY2021-K-035) and informed consent for the usage of data for research from all the enrolled HCC patients was obtained on hospital admission.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681-93. [Crossref] [PubMed]
- Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol 2020;72:262-76. [Crossref] [PubMed]
- Vitale A, Peck-Radosavljevic M, Giannini EG, et al. Personalized treatment of patients with very early hepatocellular carcinoma. J Hepatol 2017;66:412-23. [Crossref] [PubMed]
- Fan H, Zhou C, Yan J, et al. Treatment of solitary hepatocellular carcinoma up to 2 cm: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e20321. [Crossref] [PubMed]
- Imai K, Yamashita YI, Yusa T, et al. Microvascular Invasion in Small-sized Hepatocellular Carcinoma: Significance for Outcomes Following Hepatectomy and Radiofrequency Ablation. Anticancer Res 2018;38:1053-60. [Crossref] [PubMed]
- Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2019;154:209-17. [Crossref] [PubMed]
- Yao LQ, Chen ZL, Feng ZH, et al. Clinical features of recurrence after hepatic resection for early-stage hepatocellular carcinoma and long-term survival outcomes of patients with recurrence: a multi-institutional analysis. Ann Surg Oncol 2022;29:4291-303. [Crossref] [PubMed]
- Zheng J, Kuk D, Gönen M, et al. Actual 10-Year Survivors After Resection of Hepatocellular Carcinoma. Ann Surg Oncol 2017;24:1358-66. [Crossref] [PubMed]
- Kang I, Jang M, Lee JG, et al. Subclassification of Microscopic Vascular Invasion in Hepatocellular Carcinoma. Ann Surg 2021;274:e1170-8. [Crossref] [PubMed]
- Kuo FY, Liu YW, Lin CC, et al. Microscopic portal vein invasion is a powerful predictor of prognosis in patients with hepatocellular carcinoma who have undergone liver resection. J Surg Oncol 2021;123:222-35. [Crossref] [PubMed]
- Onaca N, Davis GL, Jennings LW, et al. Improved results of transplantation for hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl 2009;15:574-80. [Crossref] [PubMed]
- Ryu T, Takami Y, Wada Y, et al. A Clinical Scoring System for Predicting Microvascular Invasion in Patients with Hepatocellular Carcinoma Within the Milan Criteria. J Gastrointest Surg 2019;23:779-87. [Crossref] [PubMed]
- Bai S, Yang P, Xie Z, et al. Preoperative Estimated Risk of Microvascular Invasion is Associated with Prognostic Differences Following Liver Resection Versus Radiofrequency Ablation for Early Hepatitis B Virus-Related Hepatocellular Carcinoma. Ann Surg Oncol 2021;28:8174-85. [Crossref] [PubMed]
- Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg 2002;6:224-32; discussion 232. [Crossref] [PubMed]
- Lim KC, Chow PK, Allen JC, et al. Microvascular invasion is a better predictor of tumor recurrence and overall survival following surgical resection for hepatocellular carcinoma compared to the Milan criteria. Ann Surg 2011;254:108-13. [Crossref] [PubMed]
- Chen ZH, Zhang XP, Feng JK, et al. Actual long-term survival in hepatocellular carcinoma patients with microvascular invasion: a multicenter study from China. Hepatol Int 2021;15:642-50. [Crossref] [PubMed]
- Wang H, Wu MC, Cong WM. Microvascular invasion predicts a poor prognosis of solitary hepatocellular carcinoma up to 2 cm based on propensity score matching analysis. Hepatol Res 2019;49:344-54. [Crossref] [PubMed]
- Zhang X, Li J, Shen F, et al. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol 2018;33:347-54. [Crossref] [PubMed]
- Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 2009;137:850-5. [Crossref] [PubMed]
- Sumie S, Kuromatsu R, Okuda K, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol 2008;15:1375-82. [Crossref] [PubMed]
- Sumie S, Nakashima O, Okuda K, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol 2014;21:1002-9. [Crossref] [PubMed]
- Chen L, Chen S, Zhou Q, et al. Microvascular Invasion Status and Its Survival Impact in Hepatocellular Carcinoma Depend on Tissue Sampling Protocol. Ann Surg Oncol 2021;28:6747-57. [Crossref] [PubMed]
- Cong WM, Bu H, Chen J, et al. Practice guidelines for the pathological diagnosis of primary liver cancer: 2015 update. World J Gastroenterol 2016;22:9279-87. [Crossref] [PubMed]
- Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95:1931-7. [Crossref] [PubMed]
- Li C, Ouyang W, Yang T. The association of microvascular invasion with satellite nodule, tumor multiplicity, tumor encapsulation and resection margin of hepatocellular carcinoma. J Hepatol 2022;77:890-1. [Crossref] [PubMed]
- Lee S, Kang TW, Song KD, et al. Effect of Microvascular Invasion Risk on Early Recurrence of Hepatocellular Carcinoma After Surgery and Radiofrequency Ablation. Ann Surg 2021;273:564-71. [Crossref] [PubMed]
- Chan AWH, Zhong J, Berhane S, et al. Development of pre and post-operative models to predict early recurrence of hepatocellular carcinoma after surgical resection. J Hepatol 2018;69:1284-93. [Crossref] [PubMed]
- Sheng X, Ji Y, Ren GP, et al. A standardized pathological proposal for evaluating microvascular invasion of hepatocellular carcinoma: a multicenter study by LCPGC. Hepatol Int 2020;14:1034-47. [Crossref] [PubMed]
- Wang H, Du PC, Wu MC, et al. Postoperative adjuvant transarterial chemoembolization for multinodular hepatocellular carcinoma within the Barcelona Clinic Liver Cancer early stage and microvascular invasion. Hepatobiliary Surg Nutr 2018;7:418-28. [Crossref] [PubMed]
- Iguchi T, Shirabe K, Aishima S, et al. New Pathologic Stratification of Microvascular Invasion in Hepatocellular Carcinoma: Predicting Prognosis After Living-donor Liver Transplantation. Transplantation 2015;99:1236-42. [Crossref] [PubMed]
- Fujita N, Aishima S, Iguchi T, et al. Histologic classification of microscopic portal venous invasion to predict prognosis in hepatocellular carcinoma. Hum Pathol 2011;42:1531-8. [Crossref] [PubMed]
- Chen MY, Juengpanich S, Hu JH, et al. Prognostic factors and predictors of postoperative adjuvant transcatheter arterial chemoembolization benefit in patients with resected hepatocellular carcinoma. World J Gastroenterol 2020;26:1042-55. [Crossref] [PubMed]
- Qiu Y, Yang Y, Wang T, et al. Efficacy of Postoperative Adjuvant Transcatheter Arterial Chemoembolization in Hepatocellular Carcinoma Patients With Microscopic Portal Vein Invasion. Front Oncol 2022;12:831614. [Crossref] [PubMed]
- Sun JJ, Wang K, Zhang CZ, et al. Postoperative Adjuvant Transcatheter Arterial Chemoembolization After R0 Hepatectomy Improves Outcomes of Patients Who have Hepatocellular Carcinoma with Microvascular Invasion. Ann Surg Oncol 2016;23:1344-51.
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Alkim H, Ayaz S, Sasmaz N, et al. Hemostatic abnormalities in cirrhosis and tumor-related portal vein thrombosis. Clin Appl Thromb Hemost 2012;18:409-15. [Crossref] [PubMed]
- Lei Z, Li J, Wu D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg 2016;151:356-63. [Crossref] [PubMed]