
A combined pre- and intra-operative nomogram in evaluation of degrees of liver cirrhosis predicts post-hepatectomy liver failure: a multicenter prospective study
Highlight box
Key findings
• Four pre-operative variables (HBV-DNA level, ICG-R15, prothrombin time, and cirrhotic severity scoring), and one intra-operative variable (DSM) were independent risk factors of post-hepatectomy liver failure (PHLF).
• Both the pre-operative nomogram and the combined pre- and intra- operative nomogram were efficient in predicting PHLF.
What is known and what is new?
• What is known: adequate evaluation of degrees of liver cirrhosis is essential in surgical treatment of hepatocellular carcinoma (HCC) patients.
• What is new: this study constructed and validated a combined pre- and intra-operative nomogram based on the degrees of cirrhosis in predicting PHLF in HCC patients.
What is the implication, and what should change now?
• The pre-operative model should be used pre-operatively to identify individuals who are at high risks of developing PHLF. The combined pre- and intra-operative model should be used to make a final decision on whether to continue with hepatectomy or to carry out a lesser extent of hepatectomy.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy and the fourth leading cause of cancer-related death globally (1). Hepatectomy remains the mainstay of curative treatment for HCC patients. However, a significant proportion of patients develop HCC in a background of liver cirrhosis which significantly contributes to an increase in postoperative complications and mortalities (2). Cirrhosis is a consequence of various etiologies causing liver injury, which is often followed by diffuse hepatic fibrosis wherein the normal hepatic architecture is gradually replaced by regenerative nodules (3). There exists significant histopathological heterogeneity in the degrees of cirrhosis in patients with HCC (4). This histopathological scoring system, which is based on semiquantitative evaluation of nodular size and septal width, has widely been adopted. Several studies have shown this system to be a useful predictor of short- and long-term outcomes of hepatectomy for patients with HCC (5,6). Pre-operative liver biopsy to determine the histopathological degrees of cirrhosis is invasive, and it can occasionally lead to severe complications (7). Liver biopsy has, therefore, not been recommended as a routine in pre-operative assessment of cirrhosis. An accurate and non-invasive staging system for cirrhosis is needed. A non-invasive cirrhotic severity scoring (CSS) has previously been reported by us in predicting the degrees of cirrhosis in HCC patients. This scoring relies on four variables which are routinely measured to assess patients with HCC for hepatectomy. These variables are portal vein diameter, splenic thickness, platelet count and endoscopic degree of esophageal varices. This is a simple system with high predictive accuracy (8). Recently, an industrial device (the LX-C Shore hardness tester, Laizhou, China) was adopted in our center to directly measure liver stiffness (DSM) during open hepatectomy. The preliminary study suggested that DSM could be used as an objective indicator in staging the degrees of cirrhosis (9). However, correlations between either CSS or DSM, with histopathological stages of cirrhosis need to be determined in a large prospective multi-institutional study.
In recent decades, despite great improvements in perioperative assessments, surgical techniques, and peri-operative management, post-hepatectomy liver failure (PHLF) remains as the most severe and life-threatening complication after hepatectomy. Its incidence can reach up to 32% in some reports, with PHLF being the direct cause of death in more than half of patients (10,11). Inadequate quality of residual liver function, such as those livers with a background of cirrhosis, is an important factor that commonly leads to PHLF (12,13). A recent study indicated that the severity of liver fibrosis as measured by two-dimensional shear wave elastography is an independent risk factor for PHLF (14). However, the impact of the degrees of cirrhosis on prediction of PHLF needs to be further studied. Several predictive models of PHLF have been reported on cirrhotic patients with HCC, including the model for end-stage liver disease (MELD) score, hepatic venous pressure gradient (HVPG), indocyanine green (ICG) clearance, and liver stiffness (LS) (15-17). However, all these predictive models are not effective enough in preventing deaths from PHLF in HCC patients who undergo hepatectomy with a background of cirrhosis. Furthermore, the histopathological substages of cirrhotic severity in correlation to pre- or intra-operative scoring systems, and the impact of these scoring systems on PHLF deserve further studies.
In the current study, the effectiveness of either CSS or DSM in correlating the degrees of cirrhosis with the histopathological staging of the Laennec system, and the construction of the pre-operative and combined pre- and intra-operative nomograms based on the data obtained in this study in predicting PHLF in HCC patients after hepatectomy were studied. This article was presented in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-410/rc).
Methods
Patients
This prospective multi-institutional study was conducted at five tertiary hospitals in China. These hospitals included Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology (Wuhan), Zhongnan Hospital of Wuhan University (Wuhan), Hubei Cancer Hospital (Wuhan), Xiangyang Center Hospital (Xiangyang) and Taihe Hospital of Hubei Medical College (Shiyan). Consecutive patients with single HCC and without macrovascular invasion who underwent open hepatectomy between May 18, 2019 and Dec 19, 2020 were included in this study. The ethics committee of each hospital (Tongji Hospital, Zhongnan Hospital, Hubei Cancer Hospital, Xiangyang Center Hospital, and Taihe Hospital) approved this study (No. S1022), which was conducted in accordance with the guidelines of the Declaration of Helsinki (as revised in 2013). Informed consent from all patients was obtained before recruitment. Clinical data, including patient age, gender, and etiology of liver disease, were recorded. Pre-operative evaluation, including serological examinations, chest X-ray, abdominal ultrasonography, upper gastrointestinal endoscopy, computed tomography (CT), and/or magnetic resonance imaging (MRI) was performed within 2 weeks before surgery. Liver function was assessed by a combination of liver biochemistry, Child-Pugh classification, and indocyanine green retention rate at 15 min (ICG-R15). Three-dimensional CT volumetry was used to estimate remnant liver volumes for patients with huge tumors or large tumors adjacent to the main vessels which needed major resection. The indications for hepatectomy were (I) liver function of Child-Pugh grade A, or some patients with grade B who were planned to undergo a minor liver resection; (II) adequate residual liver volume; (III) tumor technically resectable based on evaluation by experienced liver surgeons; (IV) extent of hepatectomy was in accordance with the Makuuchi criteria based on ICG-R15 (18). Anatomical hepatectomy was performed for tumors located within a segment/section/hemiliver provided that the volume of the future liver remnant was sufficient. A parenchyma-preserving hepatectomy was performed for tumors situated peripherally, or for tumors located in unfavorable sites for anatomical resection, or patients with severely gross cirrhosis as judged by operating surgeons. A major resection was defined as resection of three or more Couinaud liver segments. Postoperative complications were defined by the Dindo-Clavien classification, with major complications being defined as grade 3 and above (19). PHLF was defined by an increased international normalized ratio with concomitant hyperbilirubinemia on or after postoperative day 5 as proposed by the International Study Group of Liver Surgery (ISGLS) (20). Bile leak is defined by ISGLS and stratified as Grade A—causing no change in the patient’s management, Grade B—resulting in intervention, but not requiring re-laparotomy or Grade C—requiring re-laparotomy (21). Postoperative mortality was defined as death within 90 days of hepatectomy. All hepatitis B surface antigen (HBsAg) positive patients received antiviral therapy before and after surgery. This multicenter prospective study was registered at Clinicaltrials.gov (https://clinicaltrials.gov/; Clinical trial number: NCT04076631).
Operative technique
The details of the operations performed have been reported previously (22). In brief, a right subcostal incision with an upward midline or a left subcostal extension was used. Operative ultrasonography was routinely done to locate the tumor and to mark the transection plane. Liver transection was carried out using a Cavitron ultrasonic surgical aspirator (CUSA), Biclamp, Harmonic Scalpel, or TissueLink, in addition to finger fracturing and clamp crushing. The Pringle’s maneuver was established for potential use before hepatectomy and only used to occlude hepatic inflows whenever it is necessary. Parenchyma-preserving hepatectomy was carried out with resection margins ≥1 cm.
Pre-operative CSS
Pre-operative CSS was based on four clinical parameters (degree of severity of esophageal varices, portal vein diameter, splenic thickness, and platelet count) for staging the severity of cirrhosis as previously reported (8). The details of CSS are shown in Table S1.
Intra-operative direct liver stiffness measurement (DSM)
Liver stiffness was measured intra-operatively using the LX-C Shore hardness tester (Laizhou, China). The device for DSM is 11.5 cm in height, and 6 cm in width. The probe is a disc with a diameter of 2.5 cm (Figure S1). The ribs do not interfere with the measuring operation and readings from the device in open surgery. The procedure involved the following steps: (I) the device was set to zero, sterilized, and then wrapped up using an aseptic transparent optical cable sleeve and ensuring that the dial reading was clearly visible and the probe could be conveniently operated; (II) the probe of the device was directly and vertically pressed onto the non-tumorous liver surface which should be away from the thin-edge parts of liver; and (III) after 3 seconds when the probe was entirely in contact with the surface of the liver, the readings were shown on the gauge. The readings were expressed in HC units. Five measurements at different parts of liver were made for each patient. The mean and standard deviation (SD) of the five measured values were calculated, and the average value was used as the final measurement for statistical analysis (9).
Histopathological assessment of degrees of cirrhosis
The histopathological degrees of cirrhosis in the non-tumorous parts of the resected liver specimens were evaluated by two experienced pathologists who were blinded to the clinical information by using the Laennec staging system (23). The degrees of cirrhosis were staged as: no cirrhosis (F0 to F3); mild cirrhosis (F4A with marked septation and rounded contours); moderate cirrhosis (F4B with at least two broad septa); and severe cirrhosis (F4C with at least one very broad septum or many minute nodules).
Statistical analysis
R Project for Statistical Computing (version 4.1.2, https://www.r-project.org/) and GraphPad Prism (version 7.00) were used for statistical analyses. Continuous variables with normal distributions were presented as mean ± standard deviation (SD). Variables which were not normally distributed were presented as median and interquartile range (IQR). Appropriate statistical tests of the Student’s t-test or Mann-Whitney U test were used. Categorical variables were expressed as frequencies or percentages, and compared with the Chi-square test or Fisher exact test. The degrees of correlation between rank variables and continuous variables were assessed by Spearman’s correlation coefficient. The diagnostic performance of CSS and DSM in assessing the severity of liver cirrhosis was determined using the receiver operating characteristic (ROC) curves. The areas under the ROC curves (AUCs) were compared using the Delong test (24). A two-tailed P value <0.05 was considered statistically significant.
In this study, the univariate and multivariate logistic regression analyses with forward stepwise were used to construct the prediction models for PHLF, using “rms” package in R. For the overall cohort, univariate logistic regression analyses were used to determine the pre-operative and intra-operative risk factors which were closely related to PHLF. Variables with a P<0.05 on univariate analysis were further included in the multivariate logistic regression analysis.
The performance ability and robustness of the models were assessed by discrimination, calibration ability, and clinical practicability for the derivation cohort (whole cohort) and validation cohort (Tongji cohort and external cohort), respectively. The Tongji cohort included patients from Tongji Hospital and the external cohort included patients from Zhongnan Hospital, Hubei Cancer Hospital, Xiangyang Center Hospital, and Taihe Hospital. The AUC or concordance index (C-index) was used to estimate the model discrimination. The Brier score was commonly used to evaluate the overall performance of a predictive model. If the predicted and actual observations of the model are relatively close, the closer the Brier score is to 0, the more perfect the overall performance of the model is (25,26). Calibration and decision curve analysis (DCA) curves were used to demonstrate the calibration capability and clinical usefulness of the models, respectively.
Results
Patient characteristics
The patient characteristics of the 327 patients who were enrolled in this study are shown in Table 1. Based on our previously reported preoperative CSS, 157 (48.0%), 70 (21.4%), 45 (13.8%), 26 (7.9%), and 29 (8.9%) patients had CSS of 0, 1, 2, 3, and ≥4, respectively. The median (IQR) intra-operative DSM was 5.7 (3.0–8.0) HC. Based on histopathological assessment, the number of patients who had no (F0-3), mild (F4A), moderate (F4B) or severe (F4C) cirrhosis were 141 (43.1%), 91 (27.8%), 71 (21.7%) and 24 (7.4%), respectively.
Table 1
| Variables | Patients (n=327) |
|---|---|
| Medical center | |
| Tongji Hospital | 178 (54.4) |
| Zhongnan Hospital | 18 (5.5) |
| Hubei Cancer Hospital | 41 (12.6) |
| Xiangyang Center Hospital | 48 (14.7) |
| Taihe Hospital | 42 (12.8) |
| Age* (years) | 55.4±10.6 |
| Gender | |
| Male | 277 (84.7) |
| Female | 50 (15.3) |
| Etiology | |
| Hepatitis B | 270 (82.6) |
| Hepatitis C | 9 (2.8) |
| Chronic alcoholism | 7 (2.1) |
| Schistosomiasis | 4 (1.2) |
| Unknown | 37 (11.3) |
| HBV-DNA (copies/mL) | |
| ≤500 | 198 (60.6) |
| >500 | 129 (39.4) |
| ICG-R15* (%) | 5.0 (2.8–7.7) |
| ALT* (U/L) | 27.0 (19.0–42.2) |
| AST* (U/L) | 30.0 (23.0–43.5) |
| ALB* (g/L) | 40.9 (38.4–44.0) |
| TBIL* (μmol/L) | 13.2 (9.95–17.6) |
| PT* (sec) | 13.6 (12.9–14.5) |
| PTA* (%) | 90.0 (72.0–98.0) |
| INR* | 1.05 (1.00–1.12) |
| WBC* (×109/L) | 5.2 (4.1–6.3) |
| RBC* (×1012/L) | 4.4 (4.1–4.8) |
| PLT* (×109/L) | 160 (113–206) |
| Tumor size* (cm) | 5.4 (3.5–7.9) |
| Child-Pugh grade | |
| A | 303 (92.7) |
| B | 24 (7.3) |
| CSS | |
| 0 | 157 (48.0) |
| 1 | 70 (21.4) |
| 2 | 45 (13.8) |
| 3 | 26 (7.9) |
| ≥4 | 29 (8.9) |
| DSM value* (HC) | 5.7 (3.0–8.0) |
| Histopathological degree of liver cirrhosis (Laennec grade) | |
| No (F0-3) | 141 (43.1) |
| Mild (F4A) | 91 (27.8) |
| Moderate (F4B) | 71 (21.7) |
| Severe (F4C) | 24 (7.4) |
Values are in n (%) or mean ± standard deviation or median (IQR). *, continuous variables. HBV, hepatitis B virus; ICG-R15, the retention rate of indocyanine green at 15 min; ALT, alanine transaminase; AST, aspartate aminotransferase; ALB, serum albumin; TBIL, total serum bilirubin; PT, prothrombin time; PTA, prothrombin activity; INR, international normalized ratio; WBC, white blood cell; RBC, red blood cell; PLT, platelet count; CSS, cirrhosis severity score; DSM, direct liver stiffness measurement; HC, the unit of measurement of the Shore hardness tester; IQR, interquartile range.
Perioperative outcomes
Details on the peri-operative data are summarized in Table 2. PHLF developed in 33 patients (10.1%), with 20 (6.1%) patients in Grade A, 10 (3.1%) patients in Grade B, and 3 (0.9%) patients in Grade C. Three (0.9%) patients died from PHLF (Table 3). The overall 30- and 90-day mortality rates were 0.9% and 0.9%, respectively.
Table 2
| Variables | Patients (n=327) |
|---|---|
| Extent of hepatectomy | |
| Minor | 229 (70.0) |
| Major | 98 (30.0) |
| Pringle’s maneuver | 230 (70.3) |
| Time of Pringle’s maneuver (min) | 11.0 [0–22.5] |
| Blood loss (mL) | 200 [100–400] |
| Blood transfusion | 39 (12.0) |
| Volume of transfused blood* (Unit) | 4.0 [2.0–4.0] |
| Patients with complications | |
| Chest infection | 10 (3.1) |
| Pleural effusion | 39 (11.9) |
| Subphrenic abscess | 5 (1.5) |
| Intra-abdominal bleeding | 5 (1.5) |
| Ascites | 59 (18.0) |
| Bile leak | 12 (3.7) |
| Severity grading of bile leak | |
| Grade A | 9 (2.8) |
| Grade B | 3 (0.9) |
| Grade C | 0 |
| Severity of complication (Clavien-Dindo) | 42 (12.8) |
| IIIa | 35 (10.7) |
| IIIb | 1 (0.3) |
| IVa | 2 (0.6) |
| IVb | 1 (0.3) |
| V | 3 (0.9) |
| Post-hepatectomy liver failure | 33 (10.1) |
| Grade A | 20 (6.1) |
| Grade B | 10 (3.1) |
| Grade C | 3 (0.9) |
| Hospital stay (days) | 12 [10–15] |
| 30-day mortality | 3 (0.9) |
| 90-day mortality | 3 (0.9) |
Values are in n (%) or median [IQR]. *, only included the 39 patients who underwent blood transfusion. IQR, interquartile range.
Table 3
| Patients | Gender | Age (years) |
ICG-R15 (%) | Tumor size (cm) | Child-Pugh grade | Extent of hepatectomy (segment) | Laennec grade | CSS score |
DSM (HC) |
Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | Male | 68 | 9.8 | 17.2 | A | I, II, III, IV, V, VIII | F4A | 3 | 7 | PHLF |
| No. 2 | Male | 59 | 3.5 | 9.3 | A | IV, V | F4B | 3 | 14 | PHLF |
| No. 3 | Male | 43 | 7.7 | 9.3 | B | IV, V | F4C | 4 | 11 | PHLF |
ICG-R15, the retention rate of indocyanine green at 15 min; CSS, cirrhosis severity score; DSM, direct liver stiffness measurement; HC, the unit of measurement of the Shore hardness tester; PHLF, post-hepatectomy liver failure.
Correlation of CSS and DSM with histopathological staging on degrees of cirrhosis
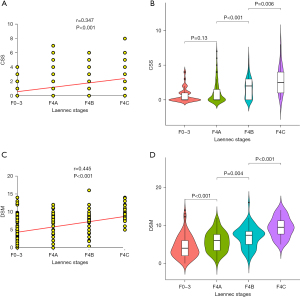
The correlation of CSS and DSM with histopathological staging on degrees of cirrhosis was showed in Figure 1. There was a significant positive correlation of CSS with histopathological staging of cirrhosis (r=0.347, P<0.001) (Figure 1A). The mean CSSs in the no (F0-3), mild (F4A), moderate (F4B), and severe (F4C) cirrhosis groups were 0.65±1.01, 1.03±1.53, 1.65±1.42, and 2.88±1.99, respectively, with an increase in CSS as the histopathological gradings increased (Figure 1B). However, there was no significant difference between the no (F0-3) and mild (F4A) cirrhosis groups. The DSM value was also significantly and positively correlated with the histopathological staging of cirrhosis (r=0.445, P<0.001) (Figure 1C). The DSM values increased significantly as the Laennec scoring system staging increased, with mean values in the no (F0-3), mild (F4A), moderate (F4B) and severe (F4C) cirrhosis groups of 4.41±2.88, 5.71±2.66, 6.96±2.83, and 9.44±2.63 HC units, respectively (Figure 1D).
Pre-operative and intra-operative prediction models for PHLF
The CSS and DSM values in relation to PHLF are shown in Figure 2. The mean values of either the CSS or the DSM in the PHLF group were significantly higher than those in the group without PHLF (all P<0.001). On univariate logistic regression analysis, six pre-operative variables [HBV-DNA level, ICG-R15, total bilirubin (TBIL), prothrombin time (PT), platelet count (PLT), Child-Pugh grade, and CSS], and five intra-operative variables (DSM, major resection, non-parenchyma-preserving hepatectomy, blood loss, and blood transfusion) were risk factors of PHLF (Table 4). On multivariate regression analysis, four preoperative variables (HBV-DNA level, ICG-R15, PT, and CSS) and one intra-operative variable (DSM) were significantly associated with PHLF (all P<0.05) (Table 4).
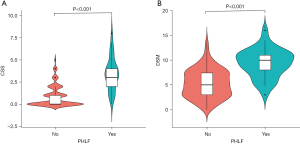
Table 4
| Variables | Univariate | Multivariate# | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Preoperative factors | |||||
| Age* (years) | 1.03 (0.99–1.06) | 0.16 | |||
| Gender | 0.30 | ||||
| Female | Reference | ||||
| Male | 1.9 (0.56–6.49) | ||||
| Etiology | 0.05 | ||||
| Others | Reference | ||||
| Hepatitis B | 7.53 (1.01–56.27) | ||||
| HBV-DNA | 0.03 | 0.04 | |||
| ≤500 copies/mL | Reference | Reference | |||
| >500 copies/mL | 2.27 (1.09–4.71) | 3.23 (1.09–10.64) | |||
| ICG-R15 | <0.001 | 0.02 | |||
| ≤10% | Reference | Reference | |||
| >10% | 4.64 (1.92–11.19) | 5.04 (1.33–18.90) | |||
| ALT* (U/L) | 1.00 (0.99–1.01) | 0.98 | |||
| AST* (U/L) | 1.00 (1.00–1.01) | 0.55 | |||
| ALB* (g/L) | 0.96 (0.89–1.04) | 0.32 | |||
| TBIL* (μmol/L) | 1.09 (1.04–1.14) | <0.001 | 1.06 (0.99–1.14) | 0.08 | |
| PT* (s) | 1.42 (1.19–1.68) | <0.001 | 1.46 (1.13–1.93) | 0.005 | |
| PTA* (%) | 1.00 (0.98–1.01) | 0.48 | |||
| INR* | 4.24 (0.60–29.96) | 0.15 | |||
| WBC* (×109/L) | 0.82 (0.66–1.02) | 0.08 | |||
| RBC* (×1012/L) | 0.91 (0.52–1.61) | 0.75 | |||
| PLT* (×109/L) | 0.99 (0.99–1.00) | 0.04 | 1.00 (1.00–1.01) | 0.31 | |
| Tumor size* (cm) | 1.08 (0.98–1.18) | 0.10 | |||
| Child-Pugh | 0.02 | 0.24 | |||
| A | Reference | ||||
| B | 3.41 (1.25–9.31) | 2.41 (0.54–1.04) | |||
| CSS* | 2.69 (2.02–3.58) | <0.001 | 2.05 (1.47–3.01) | <0.001 | |
| Intraoperative factors | |||||
| DSM value* (HC) | 1.59 (1.34–1.85) | <0.001 | 1.42 (1.17–1.75) | <0.001 | |
| Extent of hepatectomy | 0.04 | 0.71 | |||
| Minor | Reference | Reference | |||
| Major | 2.12 (1.02–4.4) | 1.24 (0.39–3.86) | |||
| PPH | 0.02 | 0.57 | |||
| No | Reference | Reference | |||
| Yes | 0.37 (0.16–0.84) | 0.72 (0.22–2.25) | |||
| Pringle’s maneuver | 0.27 | ||||
| No | Reference | ||||
| Yes | 1.64 (0.69–3.91) | ||||
| Time of Pringle’s maneuver* (min) | 1.01 (0.99–1.03) | 0.30 | |||
| Blood loss | <0.001 | 0.16 | |||
| ≤400 mL | Reference | Reference | |||
| >400 mL | 3.16 (1.45–6.88) | 2.77 (0.66–1.13) | |||
| Blood transfusion | 0.03 | 0.65 | |||
| No | Reference | Reference | |||
| Yes | 2.71 (1.13–6.54) | 0.70 (0.14–3.29) | |||
#, forward stepwise analysis; *, continuous variables. PHLF, post-hepatectomy liver failure; OR, odds ratio; CI, confidence interval; HBV, hepatitis B virus; ICG-R15, the retention rate of indocyanine green at 15 min; ALT, alanine transaminase; AST, aspartate aminotransferase; ALB, serum albumin; TBIL, total serum bilirubin; PT, prothrombin time; PTA, prothrombin activity; INR, international normalized ratio; WBC, white blood cell; RBC, red blood cell; PLT, platelet count; CSS, cirrhosis severity score; DSM, direct stiffness measurement; HC, the unit of measurement of the Shore hardness tester; PPH, parenchyma-preserving hepatectomy.
Construction, evaluation, and validation of prediction models for PHLF
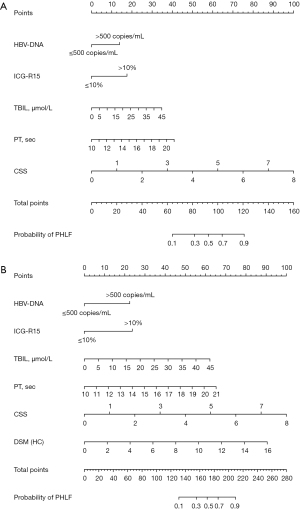
According to the forward selection based on minimizing Akaike information criterion (AIC), when the minimum AIC was 130.68, a total of six variables were selected to participate in the model construction (Figure 3). These variables were HBV-DNA level, ICG-R15, TBIL, PT, CSS, and DSM. These variables were included in the conventional model by using the pre-operative conventionally used predictors (HBV-DNA, ICG-R15, TBIL, and PT); the preoperative model by using the conventionally used preoperative predictors together with CSS (HBV-DNA, ICG-R15, TBIL, PT, and CSS), and the combined pre- and intra-operative model by using combined pre-operative predictors with intra-operative DSM (HBV-DNA, ICG-R15, TBIL, PT, CSS, and DSM). The pre-operative nomogram and the combined pre- and intra-operative nomogram are shown in Figure 3A,3B, respectively.
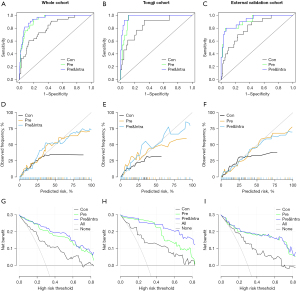
The performance ability and robustness of the three models were assessed in the whole cohort (n=327), Tongji cohort (n=178), and external validation cohort (n=149), respectively (Figure 4). The characteristics and perioperative outcomes of Tongji cohort and external cohort were shown in Table S2. The AUC curves of the models are shown in Figure 4A-4C, respectively. The AUCs of the conventional model, pre-operative model and combined pre- and intra-operative model for the whole cohort were 0.804 (95% CI: 0.729–0.879), 0.931 (95% CI: 0.897–0.965), and 0.944 (95% CI: 0.912–0.975), respectively (Figure 4A). The corresponding figures for the Tongji cohort were 0.839 (95% CI: 0.729–0.950), 0.954 (95% CI: 0.922–0.986), and 0.976 (95% CI: 0.953–0.999), respectively (Figure 4B), and for the external validation cohort were 0.800 (95% CI: 0.703–0.897), 0.914 (95% CI: 0.847–0.981), and 0.922 (95% CI: 0.861–0.984), respectively (Figure 4C). The calibration curves of the models are shown in Figure 4D-4F, respectively. For the above three cohorts, the Brier score of the combined pre- and intra-operative model was the smallest, followed by the pre-operative model and the conventional model, thus demonstrating that the accuracy of the former two models was better than the latter model. In addition, DCA showed the pre-operative model to have a stronger predictive performance than the conventional model in all the cohorts, and the combined pre- and intra-operative model had even better performance than the pre-operative model (Figure 4G-4I).
Discussion
The extent of hepatectomy is largely determined by the severity of cirrhosis, as cirrhosis significantly affects post-hepatectomy liver functional reserve (27). There are no universally accepted clinical staging systems to predict histopathological degrees of cirrhosis before hepatectomy. Surgeons often have to change the pre-operative planned strategy based on intra-operative findings on the degrees of cirrhosis (28). However, this evaluation is subjective and can differ significantly among surgeons. As a consequence, pre-operative assessment of cirrhosis becomes important in decision-makings for surgeons on HCC patients who are going to undergo hepatectomy (29). Liver biopsy for diagnosing cirrhosis has several limitations, including invasiveness, sampling error, and inter-observer variability in pathologic interpretation (30,31). The Child-Pugh classification, ICG-R15, and CT volumetry have been widely used to select optimal patients for surgery. However, for patients with a Child-Pugh grade A liver function and ICG-R15 within a normal range of <10%, the degrees of cirrhosis can vary significantly among patients (32). Although the assessments on the degrees of fibrosis have been reported on using the FibroScan, aspartate aminotransferase-to-platelet ratio index (APRI), and Fibrosis 4 score (FIB-4) (33-35), the viability of these non-invasive tests in the prediction of the severity of cirrhosis is not conclusive. Our center has focused on the use of pre-operative CSS and intra-operative DSM as tools to stage the degrees of cirrhosis in HCC patients undergoing hepatectomy. Our previously published study demonstrated a high degree of predictive accuracy of CSS on the degrees of cirrhosis (8). As DSM avoids the abdominal wall to be an interference factor as surgeons put the Shore hardness tester directly on the liver surface to measure liver stiffness, it is not surprising that when compared with the FibroScan, DSM had a significantly higher predictive accuracy in staging histopathological cirrhosis (9). In the current study, ROC analysis showed that either CSS or DSM was superior to other parameters (ARPI, ALBI, and FIB-4) in diagnosing severe cirrhosis (Figure S2).
This multicenter study demonstrated the correlations between either CSS or DSM with histopathological findings of the Laennec system in 327 HCC patients who underwent hepatectomy at five different medical institutions. An accurate determination of the degrees of cirrhosis before hepatectomy is necessary as previous studies have shown that patients with moderate/severe cirrhosis are at significantly higher risk of developing PHLF (5,36). Surgical treatment strategies need to be adjusted both pre- and intra-operatively to ensure safety of hepatectomy.
Hepatectomy for large HCC has become a safe operation in the recent few decades with acceptable peri-operative mortality (37,38). However, the removal of large tumors is frequently associated with technical difficulties and increased risk of mortality (37). Our study showed that the diameter of the tumors of the three deaths was all larger than 9 cm. In addition, the preoperative ICG-R15 was within 10%, and other parameters [such as liver function, future liver remnant (FLR), etc.] suggested that they could undergo a major resection. Nevertheless, it is worth noting that all these patients were accompanied with different degrees of cirrhosis. Although the preoperative assessment of FLR is sufficient, the function reserve of the residual liver could be significantly compromised due to cirrhosis. Therefore, in patients with large tumors and cirrhosis, more strict selection criteria for hepatectomy are required, especially emphasizing the evaluation of cirrhotic severity.
Some studies suggested that the 90-day mortality was usually higher than the 30-day mortality (39,40). The Japanese National Clinical Database showed the 30- and 90-day mortality rates after hepatectomy to be 2% and 4%, respectively (40). However, in Japan, most HCC patients who underwent hepatectomy had small tumors and were at early stages due to the effective screening program (41). In most centers outside of Japan, the majority of patients still have large HCC (>5 cm). The overall 30- and 90-day mortality rates after hepatectomy currently reported in centers outside of Japan were significantly higher than those reported in Japan (40,42). In our study, the 30- and 90-day mortality were the same (0.9%), and the 90-day mortality was lower than that of most previous studies (40). The low 90-day mortality in our study may be attributed to the following reasons: First, while many studies used ICG-R15 ≤15% as the cut-off of major resection (43), our study used ICG-R15 ≤10% as the cut-off. In the past, we encountered a number of patients with ICG-R15 ≤15% who developed PHLF after undergoing even one segmental resection in severely cirrhotic patients. This is the fundamental reason why we initiated this study focusing on evaluating the severity of cirrhosis, and multivariate analysis indeed showed that ICG-R15 >10% was a risk factor for PHLF. Second, special attention was paid to the assessment of the severity of cirrhosis both preoperatively and intraoperatively. In patients with ICG-R15 >10% and significant cirrhosis, major resection should be avoided, and parenchyma-preserving hepatectomy would be a choice, but in some cases, hepatectomy may be excluded. Effective pre-operative and intra-operative methods in assessing the degrees of cirrhosis can help surgeons to adjust strategies, which can lead to improvements in operative mortality rates.
PHLF is a potentially lethal complication after hepatectomy. It can largely be influenced by surgeons’ decisions on the extent of hepatectomy, which in many cases are based on inadequate knowledge on the degrees of cirrhosis in the background livers. In this study, 10.1% of patients developed PHLF. The 90-day mortality rate in patients with PHLF was 9.1% when compared with 0% in patients without PHLF. Although previous studies on HBV-DNA level, ICG-R15, and PT have been reported to predict PHLF (44,45), our results demonstrated the degree of cirrhosis as assessed by our reported pre-operative CSS was an additional and significant independent predictor for PHLF. Among the factors which were found in our study to be strongly correlated with PHLF, pre-operative CSS and intra-operative DSM were the most significant predictors (all P<0.001). The performance ability of the predictive models reported by us was much better than all the commonly used predictive models. Our pre-operative model should be used pre-operatively to identify individuals who are at high risks of developing PHLF. Further improvement in the predictive performance of the combined pre- and intra-operative model over the pre-operative model should be used by surgeons to make a final decision on whether to continue with hepatectomy or to carry out a lesser extent of hepatectomy. In patients with high risks of PHLF (i.e., the probability of PHLF in the models is greater than 0.5), parenchyma-preserving hepatectomy would be a choice and major resection should be avoided. Such decisions can improve the safety of surgical treatment of HCC in patients with cirrhosis.
There are several limitations of this study. First, the current intra-operative measurement device was not specifically built for this purpose, making it unsuitable for laparoscopic hepatectomy. Progress has been made with the electronic engineering team to improve the accuracy and suitability of new devices for both open and laparoscopic use. Second, the CSS and DSM are mainly used to differentiate the severity of cirrhosis and are not suitable for diagnosing the early stages of liver fibrosis (i.e., F0–F3). Third, long-term survival data are still lacking in this study. Further studies focusing on device improvements, long-term follow-up, and large-scale multicenter validation are still needed.
Conclusions
In summary, to our knowledge, this is the first prospective study to investigate the use of a combined pre- and intra-operative model in predicting PHLF after hepatectomy for HCC patients. The study demonstrated that the pre-operative model was effective in predicting PHLF in these patients, and the combined pre- and intra-operative model further improved the effectiveness.
Acknowledgments
We thank Dr. Shu Chang (Hepatic Surgery Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China) for her professional advice on the statistical analysis of this study.
Funding: This work was supported by the grants from
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-410/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-410/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The ethics committee of each hospital (Tongji Hospital, Zhongnan Hospital, Hubei Cancer Hospital, Xiangyang Center Hospital, and Taihe Hospital) approved this study (No. S1022). All the five hospitals used the same approval ID in this study. Informed consent from all patients was obtained before recruitment.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yeo YH, Hwang J, Jeong D, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol 2021;75:856-64. [Crossref] [PubMed]
- Ginès P, Krag A, Abraldes JG, et al. Liver cirrhosis. Lancet 2021;398:1359-76. [Crossref] [PubMed]
- Gu J, Zhang E, Liang B, et al. Liver Collagen Contents Are Closely Associated with the Severity of Cirrhosis and Posthepatectomy Liver Failure in Patients with Hepatocellular Carcinoma and Child-Pugh Grade A Liver Function. Ann Surg Oncol 2021;28:4227-35. [Crossref] [PubMed]
- Zhang EL, Li J, Li J, et al. Sub-Classification of Cirrhosis Affects Surgical Outcomes for Early Hepatocellular Carcinoma Independent of Portal Hypertension. Front Oncol 2021;11:671313. [Crossref] [PubMed]
- Kim SU, Oh HJ, Wanless IR, et al. The Laennec staging system for histological sub-classification of cirrhosis is useful for stratification of prognosis in patients with liver cirrhosis. J Hepatol 2012;57:556-63. [Crossref] [PubMed]
- Gill US, Pallett LJ, Kennedy PTF, et al. Liver sampling: a vital window into HBV pathogenesis on the path to functional cure. Gut 2018;67:767-75. [Crossref] [PubMed]
- Zhang EL, Zhang ZY, Wang SP, et al. Predicting the severity of liver cirrhosis through clinical parameters. J Surg Res 2016;204:274-81. [Crossref] [PubMed]
- Gu J, Zhang E, Liang B, et al. Use of Direct Liver Stiffness Measurement in Evaluating the Severity of Liver Cirrhosis in Patients with Hepatocellular Carcinoma. World J Surg 2020;44:2777-83. [Crossref] [PubMed]
- Citterio D, Facciorusso A, Sposito C, et al. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg 2016;151:846-53. [Crossref] [PubMed]
- Søreide JA, Deshpande R. Post hepatectomy liver failure (PHLF) - Recent advances in prevention and clinical management. Eur J Surg Oncol 2021;47:216-24. [Crossref] [PubMed]
- Hobeika C, Fuks D, Cauchy F, et al. Impact of cirrhosis in patients undergoing laparoscopic liver resection in a nationwide multicentre survey. Br J Surg 2020;107:268-77. [Crossref] [PubMed]
- van den Broek MA, Olde Damink SW, Dejong CH, et al. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int 2008;28:767-80. [Crossref] [PubMed]
- Long H, Zhong X, Su L, et al. Liver Stiffness Measured by Two-Dimensional Shear Wave Elastography for Predicting Symptomatic Post-hepatectomy Liver Failure in Patients with Hepatocellular Carcinoma. Ann Surg Oncol 2022;29:327-36. [Crossref] [PubMed]
- Prodeau M, Drumez E, Duhamel A, et al. An ordinal model to predict the risk of symptomatic liver failure in patients with cirrhosis undergoing hepatectomy. J Hepatol 2019;71:920-9. [Crossref] [PubMed]
- Sato N, Kenjo A, Suzushino S, et al. Predicting Post-Hepatectomy Liver Failure Using Intra-Operative Measurement of Indocyanine Green Clearance in Anatomical Hepatectomy. World J Surg 2021;45:3660-7. [Crossref] [PubMed]
- Qiu T, Fu R, Ling W, et al. Comparison between preoperative two-dimensional shear wave elastography and indocyanine green clearance test for prediction of post-hepatectomy liver failure. Quant Imaging Med Surg 2021;11:1692-700. [Crossref] [PubMed]
- Makuuchi M, Kosuge T, Takayama T, et al. Surgery for small liver cancers. Semin Surg Oncol 1993;9:298-304. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Huang ZY, Liang BY, Xiong M, et al. Severity of cirrhosis should determine the operative modality for patients with early hepatocellular carcinoma and compensated liver function. Surgery 2016;159:621-31. [Crossref] [PubMed]
- Kim MY, Cho MY, Baik SK, et al. Histological subclassification of cirrhosis using the Laennec fibrosis scoring system correlates with clinical stage and grade of portal hypertension. J Hepatol 2011;55:1004-9. [Crossref] [PubMed]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837-45.
- Martinez-Zayas G, Almeida FA, Simoff MJ, et al. A Prediction Model to Help with Oncologic Mediastinal Evaluation for Radiation: HOMER. Am J Respir Crit Care Med 2020;201:212-23. [Crossref] [PubMed]
- Pintye J, Drake AL, Kinuthia J, et al. A Risk Assessment Tool for Identifying Pregnant and Postpartum Women Who May Benefit From Preexposure Prophylaxis. Clin Infect Dis 2017;64:751-8. [Crossref] [PubMed]
- Zhang EL, Liang BY, Chen XP, et al. Severity of liver cirrhosis: a key role in the selection of surgical modality for Child-Pugh A hepatocellular carcinoma. World J Surg Oncol 2015;13:148. [Crossref] [PubMed]
- Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-50.
- Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2019;154:209-17. [Crossref] [PubMed]
- Soresi M, Giannitrapani L, Cervello M, et al. Non invasive tools for the diagnosis of liver cirrhosis. World J Gastroenterol 2014;20:18131-50. [Crossref] [PubMed]
- Rousselet MC, Michalak S, Dupré F, et al. Sources of variability in histological scoring of chronic viral hepatitis. Hepatology 2005;41:257-64. [Crossref] [PubMed]
- Gu J, Zhang E, Liang B, et al. Effectiveness comparison of indocyanine green retention test with the cirrhotic severity scoring in evaluating the pathological severity of liver cirrhosis in patients with hepatocellular carcinoma and Child-Pugh grade A liver function. World J Surg Oncol 2020;18:79. [Crossref] [PubMed]
- Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to platelet ratio index and fibrosis-4 index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology 2015;61:292-302. [Crossref] [PubMed]
- Schmoyer CJ, Kumar D, Gupta G, et al. Diagnostic Accuracy of Noninvasive Tests to Detect Advanced Hepatic Fibrosis in Patients With Hepatitis C and End-Stage Renal Disease. Clin Gastroenterol Hepatol 2020;18:2332-2339.e1. [Crossref] [PubMed]
- Foucher J, Chanteloup E, Vergniol J, et al. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut 2006;55:403-8. [Crossref] [PubMed]
- Li J, Tao HS, Li J, et al. Effect of Severity of Liver Cirrhosis on Surgical Outcomes of Hepatocellular Carcinoma After Liver Resection and Microwave Coagulation. Front Oncol 2021;11:745615. [Crossref] [PubMed]
- Wee JJ, Tee CL, Junnarkar SP, et al. Outcomes of surgical resection of super-giant (≥15 cm) hepatocellular carcinoma: Volume does matter, if not the size. J Clin Transl Res 2022;8:209-17.
- Chen XP, Qiu FZ, Wu ZD, et al. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol 2006;12:4652-5. [Crossref] [PubMed]
- Sheriff S, Madhavan S, Lei GY, et al. Predictors of mortality within the first year post-hepatectomy for hepatocellular carcinoma. J Egypt Natl Canc Inst 2022;34:14. [Crossref] [PubMed]
- Kenjo A, Miyata H, Gotoh M, et al. Risk stratification of 7,732 hepatectomy cases in 2011 from the National Clinical Database for Japan. J Am Coll Surg 2014;218:412-22. [Crossref] [PubMed]
- Kudo M, Izumi N, Kubo S, et al. Report of the 20th Nationwide follow-up survey of primary liver cancer in Japan. Hepatol Res 2020;50:15-46. [Crossref] [PubMed]
- Farges O, Goutte N, Bendersky N, et al. Incidence and risks of liver resection: an all-inclusive French nationwide study. Ann Surg 2012;256:697-704; discussion 704-5. [Crossref] [PubMed]
- Lei GY, Shen L, Junnarkar SP, et al. Predictors of 90-Day Mortality following Hepatic Resection for Hepatocellular Carcinoma. Visc Med 2021;37:102-9. [Crossref] [PubMed]
- Fang T, Long G, Wang D, et al. A Nomogram Based on Preoperative Inflammatory Indices and ICG-R15 for Prediction of Liver Failure After Hepatectomy in HCC Patients. Front Oncol 2021;11:667496. [Crossref] [PubMed]
- Yoshino K, Yoh T, Taura K, et al. A systematic review of prediction models for post-hepatectomy liver failure in patients undergoing liver surgery. HPB (Oxford) 2021;23:1311-20. [Crossref] [PubMed]


