
Clinical practice guidelines for acute-on-chronic liver failure: are we ready for reaching global consensus?
The concept of acute-on-chronic liver failure (ACLF) has gained increasing awareness during the last decade. It considers liver cirrhosis as a systemic disease where precipitating events lead to a sudden deterioration, decompensation and extrahepatic organ failures. Disease severity is determined by the number and types of organ failures and patients with ACLF have a distinct and worse prognosis than patients with acute decompensation but not fulfilling ACLF criteria (1-3). Short-term mortality rates range between 30–50% making ACLF the most severe condition patients may face in their chronic liver disease journey (1-3). It is still unknown why some patients develop an ACLF out of an acute decompensation and some recover quickly. There is currently no approved or broadly accepted standard treatment option to modify the disease course and liver transplantation remains the only curative approach. However, shortage of donor organs and numerous contraindications limit the access to transplantation to a small proportion of patients with ACLF. The complexity of treatment strategies, involving multiple organ systems and thus several different medical specialties, stresses the pressing demand for guidelines for diagnostic and therapeutic procedures in ACLF.
Bajaj together with colleagues from North America presented in the American Journal of Gastroenterology the official ACLF practice recommendations of the American College of Gastroenterology (4). The guideline panel consisted of six experts in the field of Hepatology and two guideline methodologists. They provided recommendations for questions identified by the expert panel being clinically relevant and key concept statements that summarized the current knowledge in a defined area. Authors presented the three most widely used ACLF definition from European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF; Europe), Asian-Pacific Association for the Study of the Liver (APASL; Asia), and North American Consortium for the Study of End-Stage Liver Disease (NACSELD; North America) and elaborated on their similarities, differences and pitfalls. Authors suggested to define ACLF as a “potentially reversible condition in patients with chronic liver disease with or without cirrhosis that is associated with the potential for multiple organ failure and mortality within 3 months in the absence of treatment of underlying liver disease, liver support, or liver transplantation” for the purpose of the guidelines.
While ACLF was highly debated 10 years ago, the publication of clinical guidelines indicates that this disease entity has gained global acceptance. However, there is no generally accepted pathophysiological concept, and it seems likely that the mechanisms leading to ACLF are highly individual, depending on the underlying liver disease, on the ACLF trigger but also on the type of organ system involvement (5-8). The fact that several attempts to establish ACLF disease modifying therapies such as granulocyte-colony stimulating factor (G-CSF) (9), the unselective interleukin-1 (IL-1) inhibitor Anakinra (10), or selective IL-1beta inhibitor canakinumab (11) failed in clinical studies clearly emphasizes that the complexity of the underlying pathomechanisms of this disease is still not well understood.
The clinical phenotype is characterized by organ failures and their numerous combinations define different clinical sub-cohorts and disease severities. Moreover, ACLF represents a syndrome with several disease phases, with exaggerated inflammation and/or cell death dominating the initial phase immediately after the triggering events followed by persistent organ dysfunction as the disease progresses due to a lack of regeneration and immune paralysis (5). The multitude and sequence of disease aspects raise the questions about the time point and type of intervention and whether patients were selected correctly for each individual therapy in previous studies.
The continuing lack of disease-modifying agents for ACLF is unfortunate, but it also raises the question of the extent to which the American College of Gastroenterology guidelines can provide more information about the management of patients with ACLF than what is provided in other guidelines for decompensated cirrhosis (12) and sepsis (13). And indeed, the recommendations provided in this guideline addressing the management of the most important complications of end-stage cirrhosis, such as hepatic encephalopathy (12), renal failure (12,14), alcoholic hepatitis (15), bacterial infection (12) as well as the general intensive care management (13) were addressed elsewhere and summarized here merely in the light of ACLF. In other sections, describing for instance the role of new diagnostic, and prognostic biomarkers, the use of liver assist devices and other non-surgical interventions, it is emphasized that the data is still not convincing enough to provide clear recommendations for further actions. In this respect, the guideline offers little practical guidance for the many day-to-day challenges we face in managing this syndrome (4).
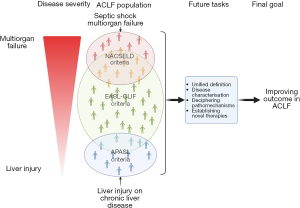
At present, there are in total 13 disease definitions of ACLF from different regions of the world with fundamental differences in disease concepts (16). The resulting heterogeneity of the disease patterns makes it almost impossible to develop globally applicable management strategies. The scientific community is therefore eagerly awaiting an unambiguous and globally applicable disease definition, based on universally accepted pathophysiological concepts, as a prerequisite for the development of therapeutic strategies to modify the course of the disease.
According to the EASL-CLIF criteria, ACLF occurs in patients with decompensated cirrhosis who develop additional organ failures. The Chronic Liver Failure Consortium Organ Failure (CLIF-C OF) score determines individual thresholds for organ failures associated with high mortality and all organs including the liver can fail (3). The NACSELD criteria were established later, initially for patients hospitalized with cirrhosis and bacterial infections. These criteria appeared to be a modification of the EASL-CLIF criteria whereby ACLF was defined by the presence of at least two extrahepatic organ failures including shock, HE grades 3 or 4, renal replacement and/or mechanical ventilation (2). The APASL criteria (1) described a more narrow disease spectrum with the liver failure in the center of the ACLF definition. The APASL ACLF definitions were extrapolated from acute liver failure, however, which develops in patients with either preexisting chronic liver disease or cirrhosis but without previous decompensation. Accordingly, patients with acute hepatic insults, which include surgical procedures, reactivation of viral hepatitis (flares) or drug-induced liver injury, but who do not have cirrhosis-related events such as infections or variceal hemorrhage, meet the APASL ACLF criteria if these events lead to jaundice and coagulopathy and are complicated by ascites or hepatic encephalopathy within 4 weeks.
The EASL-CLIF criteria, in contrast to the NACLSELD and APASL criteria, describe a relatively broad spectrum of disease severities, including early ACLF disease phases with better prognosis and higher probability of cure, as well as more advanced phases with multiple organ failure, high short-term mortality and high likelihood of futility (17). This concept is the basic requirement for differentiated treatment approaches of distinct disease stages and severities and follows a philosophy that was already successfully implemented, for example, for malignant diseases with the tumor-node-metastasis (TNM) classification, in which differentiated disease stages are defined according to their prognosis. The robustness and validity of the EASL-CLIF criteria for predicting survival by categorising patients in different ACLF severity stages was confirmed in numerous studies. The NACSELD criteria represent a patient cohort that is almost comparable to patients with septic shock where infections lead to an increase of the organ dysfunction score [Sequential Organ Failure Assessment (SOFA) score] ≥2 together with the need to vasopressor therapy to maintain the blood pressure (18), and identical to patients with very advanced stages of the EASL-CLIF ACLF definition. Therefore, the NACSELD ACLF definition does not allow discriminating between distinct disease phases and stages and the prognosis without transplantation is rather limited. Accordingly, in a comparative analysis between EASL-CLIF and NACSELD criteria by using the same cohort significantly more patients were diagnosed with ACLF by using the EASL-CLIF criteria (40% vs. 5%, P<0.001) whilst the in-hospital mortality of patients with ACLF according to the NACSELD criteria exceeded those with EASL-CLIF ACLF (30% vs. 10%, P=0.002) (19). The ACLF definition from APASL recognizes predominantly early ACLF stages. Nevertheless, the concept of segregating the ACLF cohort in sub-cohorts with similar prognosis and disease severity based on the multi-organ involvement was recently also adapted by APASL and their ACLF research consortium (AARC), which established an AARC ACLF score with three different severity categories based on the total bilirubin, hepatic encephalopathy grade, international normalized ratio (INR), serum lactate and creatinine. Using these categories, ACLF grade 1 representing patients with almost no organ failure had a low 28-day mortality rate of 13% while patients with ACLF grade 3 died in >85% (20). Although the APASL definition assumes the existence of a chronic liver disease, unlike EASL-CLIF it does not differentiate between ACLF with or without cirrhosis. However, it seems apparent that patients with underlying cirrhosis have a dismal prognosis as compared to those without cirrhosis if diagnosed based on the APASL criteria (21).
These elementary differences illustrate that results on prognosis are difficult to compare between distinct disease definitions. Consequently, the therapeutic treatment of ACLF may vary in different regions of the world, as it can involve very different disease entities. Whilst intensive care and control of infections may be the domain of NACSELD ACLF the prevention of progressing liver injury by treating hepatitis B or alcoholic hepatitis may be the therapeutic focus in APASL ACLF and renal failure and its management seems to be a frequent problem when EASL-CLIF criteria are applied (19).
Concerted effort and action are needed to tackle most of the challenges in order to establish a globally accepted ACLF disease paradigm. The American College of Gastroenterology guidelines from Bajaj et al. may be regarded as the good start for a global debate, and indeed, they attempted to identify common ground (4). Intense discussions among the different communities are essential in the future also to address some key disease aspects not only to provide a clear path for research activities but also to determine management strategies for clinicians. Agnostic approaches are required to decipher the complexity of the whole ACLF population and to segregate patients into clusters with similar phenotypes and assumed unique compositions of pathomechanisms. It will be worth aspiring to shape our future studies in a manner that novel agents will be tested in clearly defined patient subgroups matching with the assumed therapeutic target in order to maximize their efficacy and to reduce the potential side effects. Moreover, patient management will also depend on identifying patients with high likelihood of recovery and those patients where conservative therapy may be futile, unless liver transplantation is available. The large amount of data generated with the EASL-CLIF criteria is impressive but inclusion rather than exclusion of alternative concepts will be key for achieving the goal of the next decade: to reach a global consensus on how to improve the life of patients with ACLF (Figure 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-6/coif). CE reports grants from EU-Horizon 2020, Else-Kroener Fresenius and Albireo, reports royalties from Hepyx Ltd., honoraria from Albireo, Novartis, Intercept/Advanz Pharma. TB reports grants from Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Norgine, Novartis, Orphalan and Sequana Medical, consulting fees from Abbvie, Alexion, Bayer, Gilead, GSK, Eisai, Enyo Pharma, HepaRegeniX GmbH, Humedics, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Orphalan, Roche, Sequana Medical, SIRTEX, SOBI, and Shionogi, payment or honoraria from Abbvie, Alexion, Bayer, Gilead, Eisai, Falk Foundation, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, Orphalan, Sequana Medica, SIRTEX, and SOBI, support for attending meetings from Gilead, Abbvie, Intercept and Janssen. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sarin SK, Choudhury A, Sharma MK, et al. Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int 2019;13:353-90. [Crossref] [PubMed]
- O'Leary JG, Reddy KR, Garcia-Tsao G, et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018;67:2367-74. [Crossref] [PubMed]
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013;144:1426-37, 1437.e1-9.
- Bajaj JS, O'Leary JG, Lai JC, et al. Acute-on-Chronic Liver Failure Clinical Guidelines. Am J Gastroenterol 2022;117:225-52. [Crossref] [PubMed]
- Engelmann C, Clària J, Szabo G, et al. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J Hepatol 2021;75:S49-66. [Crossref] [PubMed]
- Engelmann C, Habtesion A, Hassan M, et al. Combination of G-CSF and a TLR4 inhibitor reduce inflammation and promote regeneration in a mouse model of ACLF. J Hepatol 2022;77:1325-38. [Crossref] [PubMed]
- Engelmann C, Sheikh M, Sharma S, et al. Toll-like receptor 4 is a therapeutic target for prevention and treatment of liver failure. J Hepatol 2020;73:102-12. [Crossref] [PubMed]
- Kondo T, Macdonald S, Engelmann C, et al. The role of RIPK1 mediated cell death in acute on chronic liver failure. Cell Death Dis 2021;13:5. [Crossref] [PubMed]
- Engelmann C, Herber A, Franke A, et al. Granulocyte-colony stimulating factor (G-CSF) to treat acute-on-chronic liver failure: A multicenter randomized trial (GRAFT study). J Hepatol 2021;75:1346-54. [Crossref] [PubMed]
- Szabo G, Mitchell M, McClain CJ, et al. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology 2022;76:1058-68. [Crossref] [PubMed]
- Vergis N, Patel VC, Bogdanowicz K, et al. Il-1beta Signal Inhibition in acute alcoholic hepatitis: a multicentre, randomised, double-blind, placebo-controlled phase 2 trial of canakinumab therapy (ISAIAH). J Hepatol 2022;77:S34-5. [Crossref]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol 2018;69:406-60. [Crossref] [PubMed]
- Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit Care Med 2021;49:e1063-143. [Crossref] [PubMed]
- Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol 2013;59:482-9. [Crossref] [PubMed]
- Singal AK, Bataller R, Ahn J, et al. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol 2018;113:175-94. [Crossref] [PubMed]
- Hernaez R, Solà E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut 2017;66:541-53. [Crossref] [PubMed]
- Engelmann C, Thomsen KL, Zakeri N, et al. Validation of CLIF-C ACLF score to define a threshold for futility of intensive care support for patients with acute-on-chronic liver failure. Crit Care 2018;22:254. [Crossref] [PubMed]
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:801-10. [Crossref] [PubMed]
- Wong F, Reddy KR, Tandon P, et al. The Prediction of In-Hospital Mortality in Decompensated Cirrhosis with Acute-on-Chronic Liver Failure. Liver Transpl 2022;28:560-70. [Crossref] [PubMed]
- Choudhury A, Jindal A, Maiwall R, et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol Int 2017;11:461-71. [Crossref] [PubMed]
- Chen T, Yang Z, Choudhury AK, et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol Int 2019;13:695-705. [Crossref] [PubMed]


