
Multikinase inhibitor-based immunotherapy doublet for advanced hepatocellular carcinoma: its efficacy still needs to be determined
The current landscape for advanced hepatocellular carcinoma (HCC) has evolved drastically in recent years, with the ever-increasing development of novel standard first-line therapeutic strategies (e.g., the combination of atezolizumab and bevacizumab) and the approval of several new agents for second line strategies, such as regorafenib and cabozantinib (1,2). Although a large number of treatment options are available for advanced HCC patients, multikinase inhibitor (MKI)-based immunotherapy doublet has become notably complex, especially after the publication of the COSMIC-312 trial (3). This study evaluated the efficacy and safety of cabozantinib combined with atezolizumab versus sorafenib as the first-line treatment of patients with advanced HCC. Although the primary endpoint of progression-free survival (PFS) was significantly improved with treatment using cabozantinib combined with atezolizumab compared with sorafenib in the COSMIC-312 trial, the overall survival (OS) did not improve, and the response rate was lower than expected (3). Nevertheless, this was a crucial randomized controlled trial (RCT) evaluating the efficacy of MKI-based immunotherapy doublet as the first-line systemic therapy for advanced HCC, paving the way for future investigations to determine the underlying mechanism of these connections. We have several concerns with the interpretation of this study.
Recently, MKIs have been considered promising for activating the immune checkpoint inhibitors (ICIs) response, such as cabozantinib, which is informally known as a “dirty drug” and can influence the innate and adaptative immune responses in various tumor types (4). However, besides COSMIC-312, other clinical trials did not indicate the superior activity of cabozantinib combined with ICIs for the treatment of HCC (5). Perhaps, the most likely reason is that there are too many targets of cabozantinib, mainly including vascular endothelial growth factor receptor 1–3 (VEGFR1-3), and so on (3). It is often not clear which target is effective, and some targets may hinder or counteract the synergistic effects of VEGFR1-3 targets and Programmed cell death 1 (PD-1) inhibitors. As a “tolerogenic” organ, the tumor microenvironment of HCC is characterized by immunosuppression via complex mechanisms. Therefore, effective predictive biomarkers, including cabozantinib targets, their ligands, and other plasma proteins, are urgently needed to identify HCC patients who may benefit from MKI-based immunotherapy.
Our second concern is the interpretation of the patient selection process. The clinical outcomes of patients in subgroups based on etiology vary across immunotherapy-based clinical trials. In the subgroup analysis, the OS following treatment with the combination therapy appeared to be longer than that with sorafenib alone in patients with hepatitis B virus (HBV); however, this was not found in any other subgroups with hepatitis C virus or non-viral etiology in COSMIC-312. However, the proportions of patients with HBV aetiology and the proportion enrolled in Asia were relatively lower than those in the IMbrave150 study due to lower rates of enrolment in China mainland affected by the COVID-19 pandemic (1). Therefore, etiology is likely to be the potential key factor in OS differences between these two trials. It has been indicated that the microenvironment characteristics of HBV-associated HCC may be related to favorable outcomes following cabozantinib plus atezolizumab treatment. A multidimensional analysis showed that the tumor immune microenvironment of HBV-related HCC was more immunosuppressive with the enrichment of more regulatory T cells (Treg) and CD8+ resident memory T cells versus non-viral-related HCC (6). According to a pre-clinical study, cabozantinib combined with ICIs can improve anti-tumor activity by reducing the number of CD8+PD1+ lymphocytes, Tregs and promoting circulating T-cells (7). Taken together, the above evidence indicates that cabozantinib combined with ICI is a rational combinatorial strategy for the treatment of HBV-related HCC. For clinical trials that will be in the foreseeable future, novel immunotherapeutic strategies that target unique pathways in either HBV-related HCC or non-virus-related HCC should be designed for better disease management.
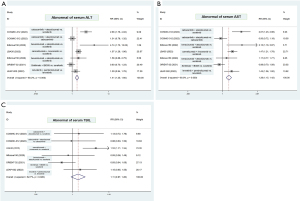
Our third concern is that therapeutic-related liver toxicity and liver functional reserve may attribute to the lack of OS improvement. In fact, inconsistent improvements in PFS and OS have been observed in several clinical trials involving patients with advanced HCC; also, toxicities including the role of therapeutic-related hepatic decompensation have increasingly been reported, posing a challenge for clinicians (8). To derive a more comprehensive estimation of the immunotherapy-based combination strategy in advanced HCC, we performed a meta-analysis of the incidence of hepatic decompensation based on phase III clinical trials, including COSMIC-312, IMbrave 150, LBA35, ORIENT-32, and LEAP-002 (Figure 1). The result showed that the frequencies of abnormal serum alanine aminotransferase (ALT) and abnormal aspartate aminotransferase (AST) were significantly higher among patients receiving immunotherapy combinations with tyrosine kinase inhibitors (TKIs) compared to those receiving a single-agent TKI (Figure 1). These data, albeit limited, support the combination use of TKIs as a promising strategy but with more expected toxicity. Considering the actual complex landscape of options, the therapeutic choice should be tailored to each advanced HCC patient based on clinical judgment, expected toxicity, and regulatory issues.
The last concern is the response evaluation criteria. Due to the subjectivity of radiological assessment and the heterogeneity between different radiological criteria, the necessity for new radiological criteria to assess tumor response has been highlighted, especially with the rapid development of the ICI-based combination era for HCC. In the COSMIC-312 trial, radiological responses were evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria, while systemic therapies were evaluated by the most relevant RCTs, such as REFLECT and IMbrave150 trials (1,9), according to the RECIST 1.1 and modified RECIST (mRECIST) criteria. In terms of HCC, the performance of the radiation pathway should be further evaluated to guide therapeutic selections. A previous study successfully developed a radiomics score to predict CD8+ T-cell infiltration using contrast-enhanced computed tomography (CT) scans to identify potential HCC patients who can benefit from immunotherapies (10). It is believed that the application of radiomics is an important supplement to improve the interpretability of the immunotherapeutic response for advanced HCC in future clinical trials—both RCTs and real-world studies.
In summary, it is undoubtedly necessary to introduce MKI-based immunotherapy doublet into treatment paradigms for advanced HCC. However, accurate biomarkers, clinical parameters, and predictive models are required to identify patients who may benefit from a given protocol.
Acknowledgments
We sincerely thank Dr. Shulin Li from MD Anderson Cancer Center for the guidance and revision of this letter.
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-58/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Galle PR, Finn RS, Qin S, et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial. Lancet Oncol 2021;22:991-1001. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Kelley RK, Rimassa L, Cheng AL, et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2022;23:995-1008. [Crossref] [PubMed]
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2021;384:829-41. [Crossref] [PubMed]
- Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 2018;379:54-63. [Crossref] [PubMed]
- Lim CJ, Lee YH, Pan L, et al. Multidimensional analyses reveal distinct immune microenvironment in hepatitis B virus-related hepatocellular carcinoma. Gut 2019;68:916-27. [Crossref] [PubMed]
- Esteban-Fabró R, Willoughby CE, Piqué-Gili M, et al. Cabozantinib Enhances Anti-PD1 Activity and Elicits a Neutrophil-Based Immune Response in Hepatocellular Carcinoma. Clin Cancer Res 2022;28:2449-60. [Crossref] [PubMed]
- Cheng AL, Qin S, Ikeda M, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 2022;76:862-73. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Liao H, Zhang Z, Chen J, et al. Preoperative Radiomic Approach to Evaluate Tumor-Infiltrating CD8(+) T Cells in Hepatocellular Carcinoma Patients Using Contrast-Enhanced Computed Tomography. Ann Surg Oncol 2019;26:4537-47. [Crossref] [PubMed]


