
Management of hepatocellular carcinoma recurrence after liver surgery and thermal ablations: state of the art and future perspectives
Introduction
Hepatocellular carcinoma (HCC) is the seventh most-common cancer worldwide, with an estimated incidence around 900 thousand cases per year, continuously increasing (1). As HCC accounts for the third most-common cause of cancer-related death, its prognosis is still poor, being related to the stage of diagnosis. The early stages can reach 5-year overall survival (OS) of 50–70%, thanks to important surgical and medical improvements (2).
Surgery represents the cornerstone treatment for HCC, including both liver transplantation (LT) and liver resections (LRs). However, the recurrence rate is a main issue, being as high as 20% after LT and 70% after LR (3). As HCC is almost always associated with liver cirrhosis, LT is ideally the best curative option. Nonetheless, because of organ shortage, there is a long waiting time carrying an high risk of dropout for tumor progression (4). Accordingly, LR and thermal ablations (TA) are currently considered the first-line treatment for HCC in patients with compensated cirrhosis according to all Western guidelines. LT is mainly reserved for patients who are not candidates for LR and TA due to impaired liver function, or for patients with poor prognostic factors on pathological examination after a previous resection (“de principe” LT) (5,6), or at recurrence after LR or TA (Salvage LT). On the other hand, for non-resectable liver disease, trans-arterial chemoembolization (TACE) represents the treatment of choice for patients with a suitable performance status (5,6). Medical therapy is only reserved to patients unable to undergo to any other treatment, and for systemic disease. For many years, the only treatment available was sorafenib, a kinase inhibitor, but more recently, thanks to an improved understanding of the molecular pathways of HCC carcinogenesis, several immunotherapies have been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) or are currently in advanced clinical trials (7,8).
Prevention and effective management of recurrence are undoubtedly the most important strategies to improve the outcomes of HCC treatment. Considerable effort has been made in the literature to investigate the risk factors for recurrence, the usefulness of adjuvant therapy to prevent it, and the management of recurrence after resection of HCC. However, due to a complex and varied scenario, many controversies exist about its management. Indeed, it is certainly clear that recurrence is the main problem of surgical treatment, and that transplantation is the best therapy for recurrent HCC after resection. However several studies involving hundreds of patients showed that LR in patients who have previously undergone resection of a single HCC, without prognostic factors of poor prognosis on pathology, had similar outcomes after recurrent hepatectomy than the first resection (9). Similar results were shown for TA, when technically feasible (10).
In short, organ shortage, the likelihood of recurrence even after a transplant, the numerous variables involved, such as the condition of the underlying liver and the different types of therapies available, make this issue extremely difficult to deal with.
The aim of this review is to summarize current strategies for the management of HCC recurrence, focusing on the different possible scenarios, as well as on future perspectives.
Incidence and patterns of recurrence
Previous studies have clarified the existence of different patterns of recurrence, with different implications (Figure 1). In particular, timing and location of recurrence were reported to be related with specific pathogenesis, risk factors and impact on prognosis.
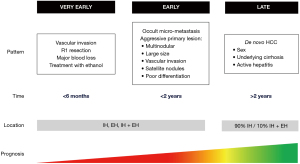
Timing and pattern of recurrence
No univocal consensus has been reached yet to define the timing of recurrence for HCC, which is telling evidence of the high stakes of this topic, but also of its complexity. HCC recurrence is classically divided into early and late recurrence according to the time to recurrence after surgery. In particular, most of the literature to date defines early recurrence as being diagnosed before 1 or 2 years (11-13). However, based on recent larger multicenter studies and our experience, we believe that 12 months is the most useful cutoff (14,15), and it should be used consistently to have a clear definition. Simon et al. in 2018 also proposed to define very early recurrence as recurrence occurring within 6 months after surgery ,which was set as the cutoff date (16). In their study on 349 patients, this pattern was associated with a worse prognosis when compared with both early and late recurrence (median OS 20.4 vs. 41.6 vs. 36.0, respectively, P<0.01). Risk factors were a treatment with ethanol, an incomplete resection (R1), a vascular invasion, and estimated blood loss higher than 1,692 mL.
Regarding to pathogenesis, early recurrence seems to originate from occult micro-metastasis of the primary tumor and is commonly associated with the aggressive characteristics of the primary lesion, such as multi-nodularity, large tumor size, poor differentiation, macro- or microvascular invasion (MVI), and satellite lesions (17,18). By contrast, late recurrence, which can occur up to 10 years after curative treatment, often has a clonal origin that is different from the original tumor, suggesting a de novo second HCC in the remnant liver. Thus, with late recurrence, some patient-related factors—such as such as sex, underlying cirrhosis and active hepatitis (19)—prevail over the characteristics of the tumor.
Location of recurrence
HCC recurrence can occur at various sites: intra-hepatically (IH), extra-hepatically (EH), and intra + extra-hepatically (I + EH). IH is the most common pattern observed after surgery, probably because of the biological tumoral characteristics, and of the high effectiveness and timeliness of surgery (20,21). As expected, IH has also the best prognosis, thanks to advances in medical and surgical therapy, as well as to the diagnostic efficacy of surveillance protocols (22). The survival rate after repeat LR or TA for HCC are similar to the outcomes after the primary hepatectomy (9). An interesting study about the prognostic meaning of location pattern was carried out by Yang et al. (21). The authors identified 3 different patterns of EH (pattern I after an initial IH; II is contemporary IH and EH; pattern III only extrahepatic), with significant differences in proportions of patients with invasion of the portal vein, hepatic vein, or inferior vena cava, intrahepatic metastases, and tumor stage between patients with intra- and extrahepatic metastases. However, the OS was the same for all the three patterns once that extrahepatic disease occurred. Thus, effective treatment for EH is very limited, except for some selected situations and for the first promising results coming from immunotherapy (23,24). It would be important to identify the risk factors of EH in order to prevent it and realize a timely surveillance and the most adapted therapy, as well as to personalize the therapy according to patterns and patients’ and tumors’ characteristics.
Finally, it is interesting to note that timing and location of HCC recurrence are correlated. An international study involving 1,004 patients showed how early recurrence was more likely to be EH or I + EH (35.5% vs. 19.8%, P=0.003), and was less likely to be treated with curative intent (33.8% vs. 45.7%, P=0.08) (25). Furthermore, compared to patients with no recurrence, patients with early recurrence had inferior OS after curative re-treatment (median OS, 69.0 vs. 140.0 months, P=0.036), which was however still better than OS in patients who received palliative treatment for early recurrence (median OS, 69.0 vs. 21.0 months, P<0.001). The cutoff for early recurrence in this study was 8 months, as identified on the basis of sensitivity analyses relative to post-recurrence survival. The overall incidence of recurrence was 44.1%, with a median recurrence-free survival time of 12 months. A paper by Xu et al. reached similar results: based on a cutoff set at 2 years, 90.1% of the patients with late recurrence were reported to have developed IH, while only 9.9%, developed I + EH (19). There were no patients who developed only EH as late recurrence.
Thus, finally, both the modality of presentation of the recurrence and the feasibility of a radical treatment are the best determinants for the prognosis (11).
Predictive factors for HCC recurrence
Identifying risk factors of recurrence is crucial to improve outcomes after the treatment of the primary HCC, in order to perform the most appropriate follow-up and the timeliest treatment. Risk factors mainly differ for early and late recurrence, and they can be divided into different categories, related to the patient, the tumor, and the treatment.
Tumor and patient related factors
Several studies have investigated risk factors for early recurrence. As mentioned above, early recurrence likely results from the occult metastasis from the initial tumor. Thus, tumor related factors indicative of high aggressiveness have been reported to be the main predictors of poor short-term oncological outcomes. In particular, most of previous studies identify as main risk factors the presence of vascular invasion (both macro-vascular and micro-vascular) and of satellite nodules, the large tumor size, multiple tumors, poor cell differentiation, and advanced pTNM stage (26-30). Recently, macrotrabecular—massive’ (MTM) HCC was described as an histological subtype, represented in about 10% of curable HCC patients and associated with tumor aggressiveness and risk of recurrence (31). Of note, most of these factors cannot be known before resection and pathologic analysis. Hence, the usefulness of preoperative criteria that can predict outcomes in HCC patients. In particular, the Milan criteria are applied to select candidates for LT, but they have also shown to be related to post-resection recurrence (32), since they include some of the aforementioned risk factors.
However, some drawbacks arise from the use of Milan Criteria, such as the absence of the consideration of the biology of the tumor. Thus, some biological markers have also been proposed as predictive factors of early recurrence. A recent study from Guo et al. investigated the role of inflammatory bio-markers in predicting early recurrence in 90 cases of HCC, finding that an elevated platelet-to-lymphocyte ratio (PLR) >103.6 was a significant independent preoperative predictor of both early recurrence (P=0.001) and OS (P=0.027) (33), while alpha-fetoprotein (AFP) was a predictive factor of only early recurrence (P=0.03). Indeed, AFP has already been proposed as a preoperative tool to predict recurrence, even though its role has mainly been investigated in the setting of LT only (34,35).
Regarding late recurrence, it is often of clonal origin and genetically different from the original tumor, suggesting a de novo second primary HCC. Thus, in this case, recurrence has been reported to be classically associated with patients-related factors, such as underlying liver conditions, including cirrhosis and active hepatitis (11,12). However, a wide multicenter study on 734 patients showed how pathologic factors of tumor aggressiveness were also important for late recurrence, with macro-vascular invasion (HR, 4.631; P<0.001), and micro-vascular invasion (HR, 1.686; P=0.001), multiple tumors (HR, 1.559; P=0.006), satellite nodules (HR, 1.587; P=0.004) and tumor size (HR, 1.487; P=0.009) found to be independent risk factors at multivariate analysis (19).
Factors related to the primary treatment
Finally, when considering factors related to the primary treatment, several studies investigated the role of the type of resection and of resection margins. Since the introduction of anatomical resection (AR) by Makuuchi in 1985, it has been proposed that the removal of the entire hepatic parenchymal tissue supplied by the portal venous system draining the lesion could result in better oncological outcomes (36). This theory was based on the concept that HCC invade the portal branches, spreading tumor cells into the portal flow, then forming satellite nodules (37). Several comparative studies were conducted about the survival benefits of AR, but their findings are still debated (38,39). Large reports showed an advantage in terms of local recurrence when comparing AR and NAR, especially for solitary tumors without MVI. A recent multi-institutional propensity-score matched study involving 250 patients with solitary HCC showed a better 5-year DFS for AR was better than for NAR (62% vs. 35%; P=0.005), although without differences in OS. This can be re-conducted to the aggressive curative-intent interventions performed after recurrence, as testified by the rate of curative repeat resection or ablation therapy between the two groups (42% vs. 10%, P=0.001), which can provide similar long term survival outcomes, even for patients undergoing NAR (40). Another multicenter study bearing on 546 patients with micro-vascular invasive HCC reported similar findings, according to which there was a lower rate of local recurrence in the site of resection after AR, even if survival outcomes were similar between the two cohorts (41). Finally, a wide meta-analysis of propensity score matching studies and randomized studies enrolling 3,554 patients also came up with 5-year survival outcomes, even in the event of a better DFS at 1- and 3-year (39). Furthermore, MVI, tumor burden and underlying liver functions were the main factors influencing long term survival.
The problem of the resection margin has also been debated, since according to Makuuchi’s theory the tumor-free margin doesn’t merely depend on the distance from the tumor (42,43). A study involving 288 patients from Poon et al. showed that the width of the resection margin did not influence the postoperative recurrence rates. Positive margins’ (R1) resection was associated with a higher HCC recurrence, but this was related to the underlying venous invasion or microsatellites (44). Thus, most intrahepatic recurrences were considered to originate from intrahepatic metastasis coming from vascular invasion, which a wide resection margin could not prevent. Similarly, Donadon et al. analyzed data from 327 consecutive patients, identifying different local recurrence rates after R0 resection (3%), R1 parenchymal (14%), R1 vascular (4%), and R1 parenchymal + R1 vascular (19%) (P=0.001) (45). The authors concluded that R1 vascular hepatectomy for HCC does not affect oncological outcomes and that detachment of hepatocellular carcinoma from intrahepatic vessels should be considered oncologically adequate.
According to Marques et al., preoperative AFP level may help determine safe margins for HCC. In the study conducted on 397 patients, surgical margins did not impact time-to-recurrence (TTR) or OS in low-AFP patients. In high-AFP group, patients with margins <1 cm had a higher recurrence rate than patients with margins ≥1 cm [(P=0.016) median TTR 8 months vs. not reported, respectively] (46).
Finally, the liver cancer study group of Japan reported interesting results from 7,964 patients, with a microscopically positive surgical margin associated with poor OS in both AR and NAR groups (47). Regarding the analysis of resection margin width between the two cohorts, AR with a negative but 0-mm surgical margin may be acceptable, while NAR with a negative 0-mm margin was associated with a less favorable survival outcome (47).
We can conclude that, while risk factors of recurrence are well established on pathology examinations, the importance of anatomical resection and of resection margin width are still debated, which calls for further perspective studies. Furthermore, the risk factors of recurrence cannot be known before surgery, suggesting the usefulness of scoring systems including tumor burden.
Monitoring after first curative treatment
Imaging monitoring modalities
The diagnostic algorithm for nodules ≥1 cm suspected for HCC in disease-free cirrhotic patients is clearly validated in international guidelines, based on non-invasive imaging criteria. European, American and Asian guidelines consensually propose biannual ultrasound screening in populations at risk of developing HCC (5,6,48). HCC diagnosis is based on imaging techniques with injection [contrast-enhanced ultrasonography (CEUS), computed tomography (CT-scan), magnetic resonance imaging (MRI)], thanks to the characteristics of tumor enhancement at the different injection times, with a typical wash-in in arterial phase and wash-out in the portal or late phase. The new Liver Imaging Reporting and Data System (LI RADS) imaging criteria are currently being validated for diagnosis by the American Association for the study of Liver Diseases (AASLD) and allow to estimate a diagnostic probability ranging from LR 1 (definitely benign lesion), to LR-5 (definitely HCC) by combining major criteria [non-rim arterial phase hyperenhancement (APHE), non-peripheral washout appearance, enhancing capsule appearance, size and threshold growth] with auxiliary criteria (49).
Imaging techniques and diagnostic modalities of recurrence after radical treatment are comparable to the initial diagnostic work-up. They are also applicable to post-therapeutic monitoring (50). According to international guidelines, the place of surveillance ranges from 2 to 4 times a year for the first two years, due to the high risk of early recurrence, regardless of the risk factors for recurrence. Subsequent lifelong monitoring should be conducted on a biannual basis, because of the risk of late recurrence, especially in case of persistent underlying liver disease. As for the type of imaging, the exact recommended modalities remain open to debate, including the monitoring by cross-sectional imaging with injection (CT or MRI), without certainty in particular as to the type of MRI contrast (extracellular or hepato-biliary) to use, even though diagnostic performance seems superior with hepato-biliary contrast agents (51). However, it seems important to alternate MRI and CT with chest sections, so as not to overlook an extrahepatic recurrence.
In addition, post-operative imaging also aims to evaluate the therapeutic efficacy of the treatment (response assessment). For thermal-ablation, according to the authors of several studies, it is performed either the day after the procedure or 4 to 6 weeks after treatment, using an injected cross-section imaging technique (CT-scan or MRI). The tumor response is evaluated by the mRECIST, which makes it possible to assess possible residual tumor viability (52-56). They also proved their reliability as an end-point for the response assessment after loco-regional treatment for phase II and III trials (57).
The Li-RADS criteria also describe an algorithm to assess response after loco-regional therapy, which is classified into three categories: Li-RADS TR Viable, equivocal or non-viable. However, the LI RADS has not yet been validated. The sensitivity of Li-RADS TR in residual tumor prediction is 40–77% for the viable category and 81–85% for the non-viable category after thermal-ablation treatment (55). Its sensitivity seems to depend on the size of the lesion. On the other hand, LI RADS has a better sensitivity for the detection of new HCC lesions (varying from 65% up to 100% for nodules >2 cm) (56). In addition, it has excellent inter-reader agreement (90%) for assessing response and diagnosing new lesions and is correlated with patient survival (57).
As for the use of PET-CT, its place remains limited in the early detection of recurrence whatever the tracer used. HCC shows a poor uptake of F18 fluor-deoxyglucose (F18FDG), responsible for the low sensitivity of F18FDG PET-CT, depending also on tumor differentiation and lesion size. F18FDG, which is less efficient than injected CT or MRI imaging techniques, is therefore recommended neither in the initial diagnosis, nor in the post-therapeutic follow-up, despite good sensitivity for the detection of extrahepatic lesions (85.7%) (58). Other markers studied, such as F-18 fluor-choline (F18-FCH), carbon-11 acetate (C11- ACT) and C-11-Choline (C11 CHOL) showed some benefits, when used alone or in combination with F18FDG, in particular with respect to the detection sensitivity of moderate or well differentiated HCC; however, they are not yet recommended in current practice (59-61).
Biomarkers dosage
The place of biomarkers is highly debated in both the initial diagnosis of HCC and in post-therapeutic monitoring.
The only biomarker routinely used in HCC screening remains AFP. However, it is not recommended by all international societies, including EASL (European association for the study of the liver): they only advise resorting to imaging screening methods, because of the poor specificity of AFP, which is responsible for false positives and possibly related to exacerbation of viral infection [hepatitis-B virus (HBV) or hepatitis-C virus (HCV)], or to hepatic decompensation. Despite this, a high preoperative rate of AFP (>400 ng/mL) appears not only as an independent risk factor for early recurrence but also as being frequently associated with MVI and as being indicative that recurrence is not eligible for transplantation, thereby reinforcing the interest in its measurement in the preoperative assessment and in its follow-up (35,62,63). The place of AFP is even less consensual in the context of post-therapeutic monitoring after radical treatment (resection or TA), in order to detect HCC recurrence, because of lower sensitivity when compared to imaging. However, it can be of interest in patients’ selection for liver transplantation after HCC recurrence. Access to transplantation under HCC is historically determined by the Milan criteria, taking into account the size and number of nodules, in order to optimize the OS and post-transplant DFS (64). Several teams have proposed the integration of the AFP rate in the selection criteria either to broaden the indications, in association with the tumor volume, or with the AFP score, as in France, to reduce the risk of post-transplant recurrence (65,66). In addition, we have shown in a previous study that the progression of the AFP score is predictive of MVI, which is a major prognostic factor of postoperative recurrence (35).
Such other markers as lectin-reactive AFP (AFP-L3) or protein induced by vitamin K absence (PIVKA II) or des-gamma-carboxy-prothrombin (DCP)—commonly used alone or in combination in particular in Asia, do not have a better accuracy in the detection of HCC at an early stage (67). The combination of AFP and AFP-L3 increases detection sensitivity to the detriment of specificity and is therefore not currently recommended in current practice at initial diagnosis and during post-therapeutic monitoring. However, a high level of pre-therapeutic PIVKA II appears to be more predictive of early recurrence than AFP (68,69). Further studies are needed to clarify the place of these biomarkers in the management of HCC.
Place of adjuvant therapy
The possibility of a postoperative treatment to reduce the incidence of tumor recurrence is a major issue in the management of hepatocellular carcinoma, because of the very high rate of post-therapeutic recurrence, affecting the prognosis. Adjuvant treatments should ideally prevent both early and late recurrence, thus acting on the identified risk factors for recurrence.
According to the results of the various randomized controlled trials conducted so far, there is currently no effective or recommended adjuvant treatment after curative treatment of HCC. Several trials investigating the role of adjuvant systemic chemotherapy, whether alone or in combination with intra-arterial chemotherapy protocols, did not demonstrate a significant increase in OS or DFS (70,71).
Intra-arterial chemotherapy alone, which has widely developed in the management of liver metastases, has not formally proved to be effective on its own in the adjuvant treatment of HCC. It has the theoretical interest of a higher concentration of chemotherapy associated with fewer systemic complications. Some previous studies with small sample size are discordant (72,73), while other monocentric RCTs seem to show a benefit on OS and DFS, especially in selected patients with MVI or satellite nodules (74-77). Larger multicenter randomized trials are needed to clarify the role of intra-arterial chemotherapy as adjuvant therapy, since current data do not allow it to be recommended.
Sorafenib was the first systemic treatment in the management of advanced HCC. However, the STORM study failed to show its efficacy as adjuvant treatment (78). In recent years, several Phase I and II trials have been published evaluating immunotherapy as monotherapy or combination in the management of advanced HCC with promising results. However, in phase III trials, only the IMBRAVE150 trial demonstrated efficacy and good safety of atezolizumab (anti-PDL1) + Bevacizumab (anti-VEGF), replacing Sorafenib in the first line of advanced HCC, opening up new perspectives for adjuvant therapy in patients at high risk of recurrence after radical treatment (79,80). There is currently not enough evidence to recommend immunotherapy as monotherapy or as adjuvant combination therapy, but several prospective international Phase III trials are currently ongoing in populations at high risk of recurrence (Table 1).
Table 1
| Trial | Included population | Immunotherapy regimen | Target | Control arm | Primary outcome | Sample size |
|---|---|---|---|---|---|---|
| CHECKMATE-9DX (NCT03383458) | Patients at high risk of recurrence after resection or ablation | Nivolumab | PD-1 | Placebo | RFS | 530 |
| KEYNOTE-937 (NCT03867084) | Patients with complete radiological response after resection or ablation | Pembrolizumab | PD-1 | Placebo | RFS, OS | 950 |
| EMERALD-2 (NCT03847428) | Patients at high risk of recurrence after resection or ablation | Durvalumab plus bevacizumab and durvalumab plus placebo | PD-L1 | Placebo plus placebo | RFS | 888 |
| IMBRAVE-050 (NCT04102098) | Patients at high risk of recurrence after resection or ablation |
Atezolizumab plus bevacizumab | PD-L1 | Active surveillance | RFS | 662 |
HCC, hepatocellular carcinoma; PD-1, programmed cell death protein 1; RFS, recurrence-free survival; OS, overall survival; PD-L1, programmed cell death ligand protein 1.
Intra-arterial treatments play an important role in HCC management, especially for intermediate and advanced stage HCC (BCLC B and C). There is currently no formal place in the recommendations for the initial treatment of very early stage or early-stage HCC (BCLC 0 and A), nor for adjuvant therapy after radical treatment. However, these treatments are studied as part of a neoadjuvant or adjuvant management of operable HCC, without strong evidence of a benefit on survival and recurrence (81). However, the analysis of two recent meta-analyses seems to suggest a benefit on overall survival and without recurrence, particularly in selected patients at high risk of recurrence (tumor volume, MVI, multinodular) (82,83). These results on large populations are however to be analyzed with caution, since there are many biases: all these studies only bore on Asian patients characterized by a high proportion of viral cirrhosis B with a high variability in surgical treatments and adjuvant treatment modalities. Further international multicenter RCTs are needed to prove the effect of adjuvant TACE. In addition, Liu et al. showed a gain in overall survival and DFS after postoperative TACE in a retrospective series of patients operated on HCC with portal thrombosis (BCLC C), thereby opening new perspectives on the place of adjuvant TACE in the context of a broadening of the resection criteria (84,85).
The development of stereotactic radiotherapy (SBRT) techniques has made it possible to provide a therapeutic alternative for patients with lesions not accessible to resection, ablation or liver transplantation. Few studies have investigated SBRT as an adjuvant therapy. The Phase II study of Chen et al. suggests good efficacy and safety in patients with a resection margin <1 cm (86). In a single-center Phase III trial, Shi et al. compared adjuvant SBRT on the resection site in patients with margins <1 cm and MVI at pathological examination: they showed that SBRT objectively improved DFS, which allowed to obtain comparable findings to recurrence rates without MVI (87). OS was also improved, although not significantly. These results suggest that adjuvant therapy has its place in patients at high risk of recurrence if confirmed by further RCTs.
The management of the underlying liver disease is essential to reduce recurrence. It includes stopping alcohol addiction, weight loss and controlling the viral disease. The advent of direct-acting antiviral drugs (DAAs) in the management of HCV was surrounded by controversy regarding its impact on the incidence and recurrence of HCC after treatment, due to several series reporting an unexpected rate of recurrence of HCC treated after DAA (88,89). Since then, several cohort studies have shown conflicting results (90,91). Recently, no increase in the risk of recurrence after treatment with DAA was reported by several meta-analyses, which even tended to demonstrate a positive effect on recurrence and survival (92). The administration timing after treatment of HCC also remains uncertain. Several RCTs are ongoing to clarify the place of DAAs in the post-therapeutic management of HCC (NCT04653818; NCT03551444). Similarly, antiviral therapy for HBV infection has showed to play an important and effective role on HCC recurrence. A recent metanalysis on 1,131 patients showed how antiviral treatment significantly reduces the rate of HBV reactivation after surgery, with a pooled risk ratio of 0.12 (P<0.00001) (93). Subsequently, it is likely that antiviral therapy can help to prevent late recurrence rather than early recurrence, due to the oncogenic role played by the HBV on the de novo carcinogenesis arising from the pathologic liver (94).
In conclusion, there is currently no data to suggest discontinuation of surveillance after treatment of viral hepatitis.
Concept of survival after recurrence
The overall survival of HCC patients is impacted both by the oncological prognosis, but also by the severity of the underlying liver pathology. In addition, HCC patients are characterized by a great heterogeneity with respect to tumor type or liver disease etiology, as frequently mentioned in the literature when referring to Western and Eastern series (95,96).
Tumor recurrence is part of the natural history of HCC evolution, with very high recurrence rates after curative treatment. Recurrence is an independent prognostic factor affecting the prognosis of patients (20,97). However, cohorts of relapsed patients associated with prolonged survival are frequently found in the literature, comparable to patients who did not experience recurrence (98-100). The recurrence of HCC, unlike other cancers, is therefore not synonymous with a terminal evolution of the pathology or with a pessimistic prognosis. Consequently, it is possible to consider the course of HCC as a chronic disease and to specifically study recurrence as a new event of its own. The future challenge is to successfully determine the risk factors of non-curable recurrence, a major event affecting the prognosis of patients with HCC. This will make it possible to select patients who can benefit from adjuvant treatments.
The outcome for the study of recidivism is paramount. It is common in oncology to reason and judge different strategies or treatments using disease free survival, or progression-free survival. Llovet et al. showed that these composite criteria had limitations since they took the two following parameters into consideration: radiological detection of recurrence and death (101). In HCC, survival is strongly influenced by the natural course of cirrhosis, which is a source of bias and potential errors in the results. Another frequently used criterion—time to recurrence—is based on the radiological detection of recurrence. It may introduce differences between groups due to possible recurrences between different imaging tests, which is a source of bias and errors in comparison between two groups.
For these reasons, it seems to us that Survival After Recurrence (SAR) is the most appropriate criterion for the study of recurrence after curative treatment. Given the natural history of HCC, SAR has a major clinical significance and appear as, a robust character. It makes it possible to specifically study the prognostic factors influencing the survival of patients at the time of recurrence and therefore to identify the predictive factors for curable recurrences and non-curable recurrences. Using SAR as a primary outcome will allow a better standardization of outcomes from an oncological perspective.
Management and curative treatments of HCC recurrence
As to therapy of recurrent HCC, many options are available, even though there is still much debate about which strategy is the best, and about the timing to follow, especially when dealing with LT. However, there is a basic assumption that guides the management of these patients: the treatment of relapse (in the absence of distant metastases) must be radical and timely. In fact, aggressive relapse therapy is able to obtain long-term results, similar to those of the treatment of the primary lesion, often even canceling any slight differences in local relapse rates after the different therapies (19).
Repeat hepatectomy (RH) vs. TAs
RH in the setting of recurrent HCC was first reported by Nagasue et al. more than 35 years ago, in a small series showing good survival outcomes and no perioperative mortality (102). Since then, thanks to the advances in both surgical techniques and perioperative care, RH is now considered a feasible option in the management of recurrent liver tumors (103,104). Indeed, a recent wide PSM study enrolling 2,689 patients showed how RH has the same OS and DFS as primary LR (105). Interestingly, at multivariate analysis MVI was the only independent prognostic factor for OS.
At the same time, further comparisons with alternative percutaneous techniques, such as TAs and other liver-based interventional and systemic therapies, were prompted by the technical difficulty due to the anatomy of a non-virgin liver, the presence of adhesions and the risk for a higher perioperative morbidity, with an increasing number of studies investigating long term outcomes (2). Indeed, in the absence of the possibility of LT, a recurrent HCC could be theoretically treated as a primary lesion, with an indication for TA in case of small and technically feasible lesions, according to main international guidelines (5,6).
A recent randomized controlled trial enrolling 240 patients from China concluded for the absence of a statistically significant difference at intention-to-treat analysis in survival outcomes after RH (1-, 3-, and 5-year OS 92.5%, 65.8% and 43.6%, respectively) compared with radiofrequency ablation (RFA) (87.5%, 52.5%, and 38.5%) in patients with early-stage recurrent HCC (106). However, RFA was associated with a higher incidence of local repeat recurrence (37.8% vs. 21.7%, P=0.04) and early repeat recurrence than RH (40.3% vs. 23.3%, P=0.04) (106). In subgroup analyses, RFA was associated with worse OS vs. repeat hepatectomy when tumor size was larger than 3 cm (HR, 1.72; 95% CI: 1.05–2.84) or with an AFP higher than 200 ng/mL (HR, 1.85; 95% CI: 1.15–2.96) (106). On the other hand, surgery had a higher complication rate than TA (22.4% vs. 7.3%, P=0.001). Similar results were recently published by Chua et al. on 219 consecutive patients with recurrent HCC, who underwent either RH or RFA. These results were analyzed by using the inverse probability of treatment weighting (IPTW), and PSM comparison (107). The minor and major postoperative complications were higher after LR than TA (30.0% and 6.0%, respectively, vs. 19.2% and 0.0%; P=0.1006). Interestingly, RH showed significantly better 3-, 5- and 10-year OS than RFA (71.3%, 59.9% and 35.4%, respectively, vs. 65.7%, 45.4% and 32.2%; P=0.04, 0.02 and 0.01). The median time to recurrence was shorter after RH (28.0 vs. 11.1 months; P=0.0225) (107).
Our experience concurs with latest literature data, suggesting some advantages in local recurrence after RH, despite similar SAR (median SAR 62 vs. 42 months, respectively; P=0.187) and the higher risk of technical demanding procedures and postoperative morbidity after RH (15). Thus, we strongly believe that accurate patients’ selection and multidisciplinary collaboration are key to the appropriate therapeutic choice.
Finally, interesting data about postoperative morbidity after RH come from the latest studies about the role in this setting of minimally invasive liver surgery. To this aim, the International Laparoscopic Liver Surgery (ILLS) society promoted a multi-institutional PSM study to compare outcomes from laparoscopic repeated hepatectomy (LRH) (108). Data from 42 centers and 1,582 patients were analyzed, showing less intraoperative blood loss after the minimally invasive approach (268 vs. 497 mL; P=0.001), with similar median OS (12.55 vs. 8.94 years; P=0.086). Of note, LRH was generally used in patients with relatively poor performance status and liver function, but favorable tumor characteristics. Similar encouraging results also came from other PSM studies, showing in some cases shorter length of hospital stay and perioperative morbidity, even if prospective trials would be needed for a final confirmation (109). Once more, patients’ selection, correlated to the center’s experience, can be the key to carry out the best treatment in such a challenging scenario.
Liver transplantation
LT can reach up to 70% of 5-year OS and similar DFS for patients with liver cirrhosis within the Milan criteria (110). Since the majority of patients develop IH recurrence with an underlying end stage liver disease, and because of the possibility with LT to remove the whole cirrhotic liver and all gross tumors, occult tumors and dysplastic nodules at the same time, survival outcomes after LT have been classically proposed to be better than partial hepatectomy (111-113). However, the severe disparity between the demand for transplantation and the supply of organs from deceased donors has precluded an expansion of the selection criteria to include patients with HCC and preserved liver function (114).
When comparing survival outcomes in patients enlisted for LT following LR before or after recurrence, de principe strategy showed better survival outcomes than salvage transplantation (4). Tribillon et al. reported results from an analysis of 111 patients showing 5-year OS rate of 84.6% versus 74.8%, respectively (P=0.017). In the multivariate analysis, the salvage strategy was the only independent prognostic factor for death [P=0.040; OR =2.5 (1.1–5.8)] (4). These results are in line with several other studies showing that salvage LT is associated with a higher operative mortality, an increased risk of recurrence, and poorer outcomes than primary LT (115,116). In addition, LR as a bridge to LT was proposed to affect the patient’s transplantability and the chance of long-term survival of cirrhotic patients with HCC (115). Primary LT should therefore remain the ideal choice of treatment of a cirrhotic patient with HCC, even when the tumor is resectable and liver function is preserved (114).
On the other hand, thanks to close surveillance protocols and multidisciplinary therapies, salvage LT has been shown in some series to be feasible in more than 60% of HCC patients after primary treatment, despite the significant recurrence after primary LR (117). Furthermore, other authors reported similar OS, DFS and postoperative complications to those related to primary LT when dealing with small HCC and compensated cirrhosis, which show how salvage LT can increase therapeutic strategies (118,119).
Thus, LR for small solitary HCC in compensated cirrhosis is still the primary option. After primary LR, Scatton et al. found that primary and recurrent HCC had the same pathological characteristics, compatible with high tumor aggressiveness (120). The authors proposed a de principe LT for patients with predictive risk of HCC recurrence, obtaining a 100% OS and DFS after a mean follow-up of 55 months. The authors concluded that this strategy could be applied in patients within the Milan criteria with poor prognosis histological features. Similarly, a high risk of failure of salvage LT was found to be associated at intention-to-treat analysis with the aforementioned pathologic risk factors of HCC recurrence (121).
Finally, HCC also recurs after LT, in a percentage of 6–16% of patients, and is often associated with poor long-term survival (122). The additional challenge in this setting is represented by the immunosuppressive therapy, with the need to balance the risk of recurrence and that of rejection. Currently, there is no consensus about the management of post-LT HCC recurrence (122). Curative treatment with LR should be considered the first-line option, showing significantly longer survival compared to that with unresectable disease (123). A minimally invasive approach has also been reported as feasible in selected cases (124). Unfortunately, only up to 30% of patients with recurrent HCC after LT are suitable for resection. Several other therapeutic approaches are available, but there are still few data. Moreover, the use of immune checkpoint inhibitors is controversial in transplant recipients considering the risk of rejection (125). Further studies are needed, with a great attention to identify risk factors and to the possible prevention of HCC recurrence.
As already stressed, the adequate selection of patients, together with the early detection of recurrence, could be the key to choose which patients should undergo de principe LT and which ones could benefit from a salvage LT, allowing the patients to reach the best prognosis, while optimizing the resource allocation in this era of organ shortage.
Future perspectives
Recurrence, which is part of the natural history of curatively treated HCC, is increased by the underlying liver disease. The literature seems to show that patients with a curable recurrence, defined by the same criteria as the initial tumor according to the BCLC classification, obtain overall survival results equivalent to patients who have not recurred. However, when the recurrence is early, very early or not curable, the impact on survival is major with poor prognosis, even if immunotherapy treatments have progressed. To improve the results of overall survival and recurrence-free survival of patients with HCC, it is necessary to:
- improve the detection of micro-metastases at the initial staging assessment and improve the surgical or thermos-ablation technique to obtain the R0;
- develop and seek the best neoadjuvant treatments for the most aggressive initial tumors. Progress will come from improving tumor aggressiveness criteria in imaging and biology and at the cellular level. It may be necessary either to reinstitute systematic tumor biopsy or to turn to new approaches based on liquid biopsies;
- await the therapeutic trial results on the surgical specimen of the adjuvant treatment by immunotherapy of patients resected from HCC at high risk of recurrence. At the time this review was written, the results of the IMBRAVE-050 trial (NCT04102098) about the efficacy of atezolizumab plus bevacizumab after liver resection have been declared as positive. This could be a real turning point in the management of curable HCC and is likely to have a huge impact on recurrence;
- maintain very close monitoring during the first 2 years in order to diagnose recurrence when itis curable and continue this monitoring beyond 5 years because late recurrences exist;
Finally, hope rests on the development of personalized medicine by genomic analysis of HCC. Even if, for the moment, the molecular classification of tumors is not applicable to clinical practice, basic and translational research is essential to allow a better understanding of the physiopathology, identify new biomarkers and propose new innovative therapies in HCC.
Each HCC is the result of a unique combination of somatic genetic alterations associated with an epigenetic profile and the dysregulation of the expression of particular genes (transcriptoma). The whole genome sequencing of tumor can be carried out quickly with limited cost thanks to high-throughput new generation sequencing (NGS). Each HCC is composed on average 40 to 60 somatic mutations and presents a large tumor genomic heterogeneity (126). These alterations include gene amplifications, mutations, insertions or deletions. They are divided into so-called “driver” mutations, involved in the carcinogenesis, and into more frequent mutations called “transients” which occur in genes not involved in carcinogenesis. The main genetic alterations and signaling pathways described in the hepatic carcinogenesis include: telomerase complex (TERT promotor 54–60%/TERC), Wnt/β Cathenin pathway, mutation of cell cycle genes (especially P53), oxydative stress pathway, modifier epigenetic genes, Ras/RAF/MapKinase pathway, ATK/mTOR, VEGF, FGF, JAK/STAT (126).
To predict the prognosis of patients treated for an HCC, more than 20 molecular signatures have been published. None of them are currently used in clinical practice (126). However, the 5-gene score (including KRT19, HN1, RAMP3, TAF9 and RAN) derived from tumor analysis published by Nault et al., predicts the occurrence of early tumor recurrence and death in HCC patients treated by liver resection, independently of the characteristics classically considered (127). Additionally, a nomogram combining the 5 gene score, MVI and the BCLC classification (Barcelona Clinic Liver Cancer) made it possible to refine the prediction of the prognosis of these patient (127). Studies have also identified from the analysis of adjacent cirrhotic tissue, molecular signatures which allowed to predict overall survival and late recurrence reflecting de novo carcinogenesis (128).
The other new approach is the liquid biopsy concept, a non-invasive, highly sensitive test first described in 2010, which enables the detection and study of tumor-derived circulating biomarkers (129). This technique should not only be very relevant for assessing tumor progression but also promising in terms of cancer disease prognosis and therapeutic follow-up (evaluation of residual cancer disease) (130). It allows the identification of tumor-derived circulating biomarkers in blood or other body fluids, such as Circulating Tumor Cells (CTCs), circulating cell-free tumor nucleic acids (ctDNA), extracellular vesicles, or tumor-educated platelets (TEPS) (130). CTCs and ctDNA may offer important information on tumor aggressiveness, especially micro-vascular invasion (131,132). Preoperative CTC positivity was found to be linked with the presence of MVI in a large cohort of 309 patients, according to Zhou et al. suggesting that CTCs could provide important information on the risk of recurrence (132). More recently, Zhao et al. have evaluated both ctDNA detection, using a personalized panel based on tumor whole exome sequencing, and CTC enumeration to detect minimal residual disease (133). They showed that postoperative the presence of CTCs (P=0.0223), ctDNA (P<0.0001) were independent risk factors of HCC recurrence. Also, three meta-analyses have studied the role of CTCs detection on clinical outcomes in HCC patients and confirmed all the prognostic value of blood CTC positivity (134-136). Indeed, the presence of CTC, whatever the CTC detection method used, was associated with decreased RFS (HR, 3.03; 95% CI: 1.89–4.86; P<0.00001) and OS (HR, 2.45; 95% CI: 1.73–3.48; P<0.0001 and HR, 2.31; 95% CI: 1.55–3.42; P<0.01) (134-136). Finally, CTC count has been used as an endpoint to evaluate the risk of dissemination of tumor cells during surgery. Hao et al. observed that “no-touch” surgery (so called anterior approach) lowered the dissemination risk of tumor cells, especially in large tumors, compared to the classical approach. As a result, the classical approach was considered as an independent RFS and OS risk factor (137).
Conclusions
In conclusion, recurrence is part of the natural history of curatively treated HCC, increased by the underlying liver disease and the spread of micro-metastases, making it difficult to obtain a complete eradication of the disease. Several therapeutic strategies are possible, which may be combined or sequential. Therapeutic decisions must be made in a multidisciplinary meeting to best suit the patient with HCC. Immunotherapy is likely to revolutionize the outcomes of HCC resected patients at high risk of recurrence. Despite a large tumor genomic heterogeneity in HCC, translational research (transcriptoma and circulating cell) is making daily progress towards the identification of molecular signatures to predict tumor aggressiveness. These different results and applications in clinical practice are encouraging and open up a promising avenue for the development of personalized treatments by precision medicine to ultimately improve survival in patients with HCC.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-579/coif). B.G. and F.P. serve as the unpaid editorial board members of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2021;7:6. [Crossref] [PubMed]
- Graf D, Vallböhmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med 2014;25:430-7. [Crossref] [PubMed]
- Tribillon E, Barbier L, Goumard C, et al. When Should We Propose Liver Transplant After Resection of Hepatocellular Carcinoma? A Comparison of Salvage and De Principe Strategies. J Gastrointest Surg 2016;20:66-76; discussion 76. [Crossref] [PubMed]
- Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018;67:358-80. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice; . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 2018;69:182-236. [Crossref] [PubMed]
- Sangro B, Sarobe P, Hervás-Stubbs S, et al. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 2021;18:525-43. [Crossref] [PubMed]
- Bruix J, Chan SL, Galle PR, et al. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960-74. [Crossref] [PubMed]
- Minagawa M, Makuuchi M, Takayama T, et al. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 2003;238:703-10. [Crossref] [PubMed]
- Wei F, Huang Q, Zhou Y, et al. Radiofrequency ablation versus repeat hepatectomy in the treatment of recurrent hepatocellular carcinoma in subcapsular location: a retrospective cohort study. World J Surg Oncol 2021;19:175. [Crossref] [PubMed]
- Portolani N, Coniglio A, Ghidoni S, et al. Early and Late Recurrence After Liver Resection for Hepatocellular Carcinoma. Ann Surg 2006;243:229-35. [Crossref] [PubMed]
- Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol 2003;38:200-7. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg 2004;240:698-708; discussion 708-10. [Crossref] [PubMed]
- Xing H, Zhang WG, Cescon M, et al. Defining and predicting early recurrence after liver resection of hepatocellular carcinoma: a multi-institutional study. HPB (Oxford) 2020;22:677-89. [Crossref] [PubMed]
- Toubert C, Guiu B, Al Taweel B, et al. Prolonged Survival after Recurrence in HCC Resected Patients Using Repeated Curative Therapies: Never Give Up! Cancers (Basel) 2022;15:232. [Crossref] [PubMed]
- Simon R, Sasaki K, Margonis GA, et al. Risk factors for very early recurrence of hepatocellular carcinoma: a retrospective review. HPB 2018;20:S83-S84. [Crossref]
- Hong YM, Cho M, Yoon KT, et al. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol 2017;39:1010428317720863. [Crossref] [PubMed]
- Cheng Z, Yang P, Qu S, et al. Risk factors and management for early and late intrahepatic recurrence of solitary hepatocellular carcinoma after curative resection. HPB (Oxford) 2015;17:422-7. [Crossref] [PubMed]
- Xu XF, Xing H, Han J, et al. Risk Factors, Patterns, and Outcomes of Late Recurrence After Liver Resection for Hepatocellular Carcinoma: A Multicenter Study From China. JAMA Surg 2019;154:209-17. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Intrahepatic recurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg 1999;229:216-22. [Crossref] [PubMed]
- Yang Y, Nagano H, Ota H, et al. Patterns and clinicopathologic features of extrahepatic recurrence of hepatocellular carcinoma after curative resection. Surgery 2007;141:196-202. [Crossref] [PubMed]
- Yoh T, Seo S, Taura K, et al. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann Surg 2021;273:792-9. [Crossref] [PubMed]
- Nakano S, Eso Y, Okada H, et al. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers (Basel) 2020;12:775. [Crossref] [PubMed]
- Arora S, Harmath C, Catania R, et al. Hepatocellular carcinoma: metastatic pathways and extra-hepatic findings. Abdom Radiol (NY) 2021;46:3698-707. [Crossref] [PubMed]
- Wei T, Zhang XF, Bagante F, et al. Early Versus Late Recurrence of Hepatocellular Carcinoma After Surgical Resection Based on Post-recurrence Survival: an International Multi-institutional Analysis. J Gastrointest Surg 2021;25:125-33. [Crossref] [PubMed]
- Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg 2000;232:10-24. [Crossref] [PubMed]
- Huang L, Li J, Yan J, et al. Early recurrence after curative resection in oligonodular hepatocellular carcinoma. Hepatogastroenterology 2013;60:28-31. [Crossref] [PubMed]
- Lee HY, Rhim H, Lee MW, et al. Early diffuse recurrence of hepatocellular carcinoma after percutaneous radiofrequency ablation: analysis of risk factors. Eur Radiol 2013;23:190-7. [Crossref] [PubMed]
- Zhou YM, Yang JM, Li B, et al. Risk factors for early recurrence of small hepatocellular carcinoma after curative resection. Hepatobiliary Pancreat Dis Int 2010;9:33-7.
- Shimoda M, Tago K, Shiraki T, et al. Risk Factors for Early Recurrence of Single Lesion Hepatocellular Carcinoma After Curative Resection. World J Surg 2016;40:2466-71. [Crossref] [PubMed]
- Ziol M, Poté N, Amaddeo G, et al. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology 2018;68:103-12. [Crossref] [PubMed]
- Lurje G, Bednarsch J, Czigany Z, et al. Prognostic factors of disease-free and overall survival in patients with hepatocellular carcinoma undergoing partial hepatectomy in curative intent. Langenbecks Arch Surg 2018;403:851-61. [Crossref] [PubMed]
- Guo Y, Chua DW, Koh YX, et al. Preoperative Predictors Including the Role of Inflammatory Indices in Predicting Early Recurrence After Re-resection for Recurrent Hepatocellular Carcinoma. World J Surg 2019;43:2587-94. [Crossref] [PubMed]
- Vibert E, Azoulay D, Hoti E, et al. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant 2010;10:129-37. [Crossref] [PubMed]
- Herrero A, Boivineau L, Cassese G, et al. Progression of AFP SCORE is a Preoperative Predictive Factor of Microvascular Invasion in Selected Patients Meeting Liver Transplantation Criteria for Hepatocellular Carcinoma. Transpl Int 2022;35:10412. [Crossref] [PubMed]
- Pang YY. The Brisbane 2000 terminology of liver anatomy and resections. HPB 2000; 2:333-39. HPB (Oxford) 2002;4:99-author reply 99-100. [Crossref] [PubMed]
- Okusaka T, Okada S, Ueno H, et al. Satellite lesions in patients with small hepatocellular carcinoma with reference to clinicopathologic features. Cancer 2002;95:1931-7. [Crossref] [PubMed]
- Famularo S, Di Sandro S, Giani A, et al. Long-term oncologic results of anatomic vs. parenchyma-sparing resection for hepatocellular carcinoma. A propensity score-matching analysis. Eur J Surg Oncol 2018;44:1580-7. [Crossref] [PubMed]
- Famularo S, Ceresoli M, Giani A, et al. Is It Just a Matter of Surgical Extension to Achieve the Cure of Hepatocarcinoma? A Meta-Analysis of Propensity-Matched and Randomized Studies for Anatomic Versus Parenchyma-Sparing Liver Resection. J Gastrointest Surg 2021;25:94-103. [Crossref] [PubMed]
- Minagawa M, Mise Y, Omichi K, et al. Anatomic Resection for Hepatocellular Carcinoma: Prognostic Impact Assessed from Recurrence Treatment. Ann Surg Oncol 2022;29:913-21. [Crossref] [PubMed]
- Hidaka M, Eguchi S, Okuda K, et al. Impact of Anatomical Resection for Hepatocellular Carcinoma With Microportal Invasion (vp1): A Multi-institutional Study by the Kyushu Study Group of Liver Surgery. Ann Surg 2020;271:339-46. [Crossref] [PubMed]
- Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet 1985;161:346-50.
- Liu H, Hu FJ, Li H, et al. Anatomical vs nonanatomical liver resection for solitary hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastrointest Oncol 2021;13:1833-46. [Crossref] [PubMed]
- Poon RTP, Fan ST, Ng IOL, et al. Significance of Resection Margin in Hepatectomy for Hepatocellular Carcinoma. Ann Surg 2000;231:544-51. [Crossref] [PubMed]
- Donadon M, Terrone A, Procopio F, et al. Is R1 vascular hepatectomy for hepatocellular carcinoma oncologically adequate? Analysis of 327 consecutive patients. Surgery 2019;165:897-904. [Crossref] [PubMed]
- Marques F, Ghallab M, Vibert E, et al. Prognostic impact of surgical margins for hepatocellular carcinoma according to preoperative alpha-fetoprotein level. HPB (Oxford) 2022;24:848-56. [Crossref] [PubMed]
- Aoki T, Kubota K, Hasegawa K, et al. Significance of the surgical hepatic resection margin in patients with a single hepatocellular carcinoma. Br J Surg 2020;107:113-20. [Crossref] [PubMed]
- Omata M, Cheng AL, Kokudo N, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int 2017;11:317-70. [Crossref] [PubMed]
- Tang A, Bashir MR, Corwin MT, et al. Evidence Supporting LI-RADS Major Features for CT- and MR Imaging-based Diagnosis of Hepatocellular Carcinoma: A Systematic Review. Radiology 2018;286:29-48. [Crossref] [PubMed]
- Rimola J, Forner A, Sapena V, et al. Performance of gadoxetic acid MRI and diffusion-weighted imaging for the diagnosis of early recurrence of hepatocellular carcinoma. Eur Radiol 2020;30:186-94. [Crossref] [PubMed]
- Mendiratta-Lala M, Masch WR, Shampain K, et al. MRI Assessment of Hepatocellular Carcinoma after Local-Regional Therapy: A Comprehensive Review. Radiol Imaging Cancer 2020;2:e190024. [Crossref] [PubMed]
- Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 2010;33:11-7. [Crossref] [PubMed]
- Nahon P, Aubé C, Moga L, et al. Non-invasive diagnosis and follow-up of primary malignant liver tumours. Clin Res Hepatol Gastroenterol 2022;46:101766. [Crossref] [PubMed]
- Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol 2020;72:288-306. [Crossref] [PubMed]
- Chaudhry M, McGinty KA, Mervak B, et al. The LI-RADS Version 2018 MRI Treatment Response Algorithm: Evaluation of Ablated Hepatocellular Carcinoma. Radiology 2020;294:320-6. [Crossref] [PubMed]
- Cools KS, Moon AM, Burke LMB, et al. Validation of the Liver Imaging Reporting and Data System Treatment Response Criteria After Thermal Ablation for Hepatocellular Carcinoma. Liver Transpl 2020;26:203-14. [Crossref] [PubMed]
- Zhang Y, Wang J, Li H, et al. Performance of LI-RADS version 2018 CT treatment response algorithm in tumor response evaluation and survival prediction of patients with single hepatocellular carcinoma after radiofrequency ablation. Ann Transl Med 2020;8:388. [Crossref] [PubMed]
- Park JW, Kim JH, Kim SK, et al. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med 2008;49:1912-21. [Crossref] [PubMed]
- Castilla-Lièvre MA, Franco D, Gervais P, et al. Diagnostic value of combining 11C-choline and 18F-FDG PET/CT in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 2016;43:852-9. [Crossref] [PubMed]
- Bieze M, Klümpen HJ, Verheij J, et al. Diagnostic accuracy of (18) F-methylcholine positron emission tomography/computed tomography for intra- and extrahepatic hepatocellular carcinoma. Hepatology 2014;59:996-1006. [Crossref] [PubMed]
- Yamamoto Y, Nishiyama Y, Kameyama R, et al. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med 2008;49:1245-8. [Crossref] [PubMed]
- Zhang Y, Lei X, Xu L, et al. Preoperative and postoperative nomograms for predicting early recurrence of hepatocellular carcinoma without macrovascular invasion after curative resection. BMC Surg 2022;22:233. [Crossref] [PubMed]
- Gelli M, Sebagh M, Porcher R, et al. Liver Resection for Early Hepatocellular Carcinoma: Preoperative Predictors of Non Transplantable Recurrence and Implications for Treatment Allocation. Ann Surg 2020;272:820-6. [Crossref] [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [Crossref] [PubMed]
- Toso C, Meeberg G, Hernandez-Alejandro R, et al. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology 2015;62:158-65. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5. [Crossref] [PubMed]
- Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009;137:110-8. [Crossref] [PubMed]
- Choi J, Kim GA, Han S, et al. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early-Stage Hepatocellular Carcinoma. Hepatology 2019;69:1983-94. [Crossref] [PubMed]
- Wang SY, Su TH, Chen BB, et al. Prothrombin induced by vitamin K absence or antagonist-II (PIVKA-II) predicts complete responses of transarterial chemoembolization for hepatocellular carcinoma. J Formos Med Assoc 2022;121:1579-87. [Crossref] [PubMed]
- Zhong J, Xiang B, Ma L, et al. Conventional oral systemic chemotherapy for postoperative hepatocellular carcinoma: A systematic review. Mol Clin Oncol 2014;2:1091-6. [Crossref] [PubMed]
- Schwartz JD, Schwartz M, Mandeli J, et al. Neoadjuvant and adjuvant therapy for resectable hepatocellular carcinoma: review of the randomised clinical trials. Lancet Oncol 2002;3:593-603. [Crossref] [PubMed]
- Kim DY, Ahn SH, Kim SU, et al. Adjuvant hepatic arterial infusional chemotherapy with 5-fluorouracil and cisplatin after curative resection of hepatocellular carcinoma. Oncology 2011;81:184-91. [Crossref] [PubMed]
- Hsiao JH, Tsai CC, Liang TJ, et al. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment. Int J Surg 2017;45:35-41. [Crossref] [PubMed]
- Huang SX, Wu YL, Tang CW, et al. Prophylactic hepatic artery infusion chemotherapy improved survival after curative resection in patients with hepatocellular carcinoma. Hepatogastroenterology 2015;62:122-5.
- Hirokawa F, Komeda K, Taniguchi K, et al. Is Postoperative Adjuvant Transcatheter Arterial Infusion Therapy Effective for Patients with Hepatocellular Carcinoma who Underwent Hepatectomy? A Prospective Randomized Controlled Trial. Ann Surg Oncol 2020;27:4143-52. [Crossref] [PubMed]
- Li S, Mei J, Wang Q, et al. Postoperative Adjuvant Transarterial Infusion Chemotherapy with FOLFOX Could Improve Outcomes of Hepatocellular Carcinoma Patients with Microvascular Invasion: A Preliminary Report of a Phase III, Randomized Controlled Clinical Trial. Ann Surg Oncol 2020;27:5183-90. [Crossref] [PubMed]
- Nitta H, Beppu T, Imai K, et al. Adjuvant hepatic arterial infusion chemotherapy after hepatic resection of hepatocellular carcinoma with macroscopic vascular invasion. World J Surg 2013;37:1034-42. [Crossref] [PubMed]
- Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 2015;16:1344-54. [Crossref] [PubMed]
- Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 2020;382:1894-905. [Crossref] [PubMed]
- Hack SP, Spahn J, Chen M, et al. IMbrave 050: a Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol 2020;16:975-89. [Crossref] [PubMed]
- Samuel M, Chow PK, Chan Shih-Yen E, et al. Neoadjuvant and adjuvant therapy for surgical resection of hepatocellular carcinoma. Cochrane Database Syst Rev 2009;2009:CD001199. [Crossref] [PubMed]
- Esagian SM, Kakos CD, Giorgakis E, et al. Adjuvant Transarterial Chemoembolization Following Curative-Intent Hepatectomy Versus Hepatectomy Alone for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers (Basel) 2021;13:2984. [Crossref] [PubMed]
- Shen A, Liu M, Zheng D, et al. Adjuvant transarterial chemoembolization after curative hepatectomy for hepatocellular carcinoma with microvascular invasion: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2020;44:142-54. [Crossref] [PubMed]
- Liu S, Guo L, Li H, et al. Postoperative Adjuvant Trans-Arterial Chemoembolization for Patients with Hepatocellular Carcinoma and Portal Vein Tumor Thrombus. Ann Surg Oncol 2018;25:2098-104. [Crossref] [PubMed]
- Zhong C, Guo RP, Li JQ, et al. A randomized controlled trial of hepatectomy with adjuvant transcatheter arterial chemoembolization versus hepatectomy alone for Stage III A hepatocellular carcinoma. J Cancer Res Clin Oncol 2009;135:1437-45. [Crossref] [PubMed]
- Chen B, Wu JX, Cheng SH, et al. Phase 2 Study of Adjuvant Radiotherapy Following Narrow-Margin Hepatectomy in Patients With HCC. Hepatology 2021;74:2595-604. [Crossref] [PubMed]
- Shi C, Li Y, Geng L, et al. Adjuvant stereotactic body radiotherapy after marginal resection for hepatocellular carcinoma with microvascular invasion: A randomised controlled trial. Eur J Cancer 2022;166:176-84. [Crossref] [PubMed]
- Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol 2016;65:719-26. [Crossref] [PubMed]
- Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol 2016;65:727-33. [Crossref] [PubMed]
- ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Electronic address: stanislas.pol@aphp. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65:734-40. [Crossref] [PubMed]
- Singal AG, Rich NE, Mehta N, et al. Direct-Acting Antiviral Therapy Not Associated With Recurrence of Hepatocellular Carcinoma in a Multicenter North American Cohort Study. Gastroenterology 2019;156:1683-1692.e1. [Crossref] [PubMed]
- Sapena V, Enea M, Torres F, et al. Hepatocellular carcinoma recurrence after direct-acting antiviral therapy: an individual patient data meta-analysis. Gut 2022;71:593-604. [Crossref] [PubMed]
- Liu KX, Hong JG, Wu R, et al. Clinical Benefit of Antiviral Agents for Hepatocellular Carcinoma Patients With Low Preoperative HBV-DNA Loads Undergoing Curative Resection: A Meta-Analysis. Front Oncol 2021;11:605648. [Crossref] [PubMed]
- Fung J, Chok KSH. The role of oral antiviral therapy in hepatitis B-related hepatocellular carcinoma. Hepatoma Res 2017;3:284-93.
- Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016;122:3430-46. [Crossref] [PubMed]
- Song TJ, Fong Y, Cho SJ, et al. Comparison of hepatocellular carcinoma in American and Asian patients by tissue array analysis. J Surg Oncol 2012;106:84-8. [Crossref] [PubMed]
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2015;261:947-55. [Crossref] [PubMed]
- Tranchart H, Chirica M, Sepulveda A, et al. Long-term outcomes following aggressive management of recurrent hepatocellular carcinoma after upfront liver resection. World J Surg 2012;36:2684-91. [Crossref] [PubMed]
- Shimada K, Sano T, Sakamoto Y, et al. A long-term follow-up and management study of hepatocellular carcinoma patients surviving for 10 years or longer after curative hepatectomy. Cancer 2005;104:1939-47. [Crossref] [PubMed]
- Nanashima A, Tanoue Y, Hiyoshi M, et al. Prognostic Value of Repeat Hepatectomy for Hepatocellular Carcinoma Patients. Anticancer Res 2022;42:4553-61. [Crossref] [PubMed]
- Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst 2008;100:698-711. [Crossref] [PubMed]
- Nagasue N, Yukaya H, Ogawa Y, et al. Second hepatic resection for recurrent hepatocellular carcinoma. Br J Surg 1986;73:434-8. [Crossref] [PubMed]
- Fan ST, Mau Lo C, Poon RT, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg 2011;253:745-58. [Crossref] [PubMed]
- Poon RT, Fan ST, Lo CM, et al. Improving survival results after resection of hepatocellular carcinoma: a prospective study of 377 patients over 10 years. Ann Surg 2001;234:63-70. [Crossref] [PubMed]
- Iaria M, Bianchi G, Fazio F, et al. The largest western experience on salvage hepatectomy for recurrent hepatocellular carcinoma: propensity score-matched analysis on behalf of He.RC.O.Le.Study Group. HPB (Oxford) 2022;24:1291-304. [Crossref] [PubMed]
- Xia Y, Li J, Liu G, et al. Long-term Effects of Repeat Hepatectomy vs Percutaneous Radiofrequency Ablation Among Patients With Recurrent Hepatocellular Carcinoma: A Randomized Clinical Trial. JAMA Oncol 2020;6:255-63. [Crossref] [PubMed]
- Chua DW, Koh YX, Syn NL, et al. Repeat hepatectomy versus radiofrequency ablation in management of recurrent hepatocellular carcinoma: an average treatment effect analysis. Ann Surg Oncol 2021;28:7731-40. [Crossref] [PubMed]
- Morise Z, Aldrighetti L, Belli G, et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: a multicentre propensity score-based study. Br J Surg 2020;107:889-95. [Crossref] [PubMed]
- Shen Z, Cai J, Gao J, et al. Efficacy of laparoscopic repeat hepatectomy compared with open repeat hepatectomy: a single-center, propensity score matching study. World J Surg Oncol 2022;20:197. [Crossref] [PubMed]
- Chan DL, Morris DL, Chua TC. Clinical efficacy and predictors of outcomes of repeat hepatectomy for recurrent hepatocellular carcinoma - a systematic review. Surg Oncol 2013;22:e23-30. [Crossref] [PubMed]
- Yamamoto J, Iwatsuki S, Kosuge T, et al. Should hepatomas be treated with hepatic resection or transplantation? Cancer 1999;86:1151-8. [Crossref] [PubMed]
- Bigourdan JM, Jaeck D, Meyer N, et al. Small hepatocellular carcinoma in Child A cirrhotic patients: hepatic resection versus transplantation. Liver Transpl 2003;9:513-20. [Crossref] [PubMed]
- Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology 1999;30:1434-40. [Crossref] [PubMed]
- Santopaolo F, Lenci I, Milana M, et al. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J Gastroenterol 2019;25:2591-602. [Crossref] [PubMed]
- Adam R, Azoulay D, Castaing D, et al. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg 2003;238:508-18; discussion 518-9. [Crossref] [PubMed]
- Bhangui P, Allard MA, Vibert E, et al. Salvage Versus Primary Liver Transplantation for Early Hepatocellular Carcinoma: Do Both Strategies Yield Similar Outcomes? Ann Surg 2016;264:155-63. [Crossref] [PubMed]
- Cherqui D, Laurent A, Mocellin N, et al. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg 2009;250:738-46. [Crossref] [PubMed]
- Vennarecci G, Ettorre GM, Antonini M, et al. First-line liver resection and salvage liver transplantation are increasing therapeutic strategies for patients with hepatocellular carcinoma and child a cirrhosis. Transplant Proc 2007;39:1857-60. [Crossref] [PubMed]
- Del Gaudio M, Ercolani G, Ravaioli M, et al. Liver transplantation for recurrent hepatocellular carcinoma on cirrhosis after liver resection: University of Bologna experience. Am J Transplant 2008;8:1177-85. [Crossref] [PubMed]
- Scatton O, Zalinski S, Terris B, et al. Hepatocellular carcinoma developed on compensated cirrhosis: resection as a selection tool for liver transplantation. Liver Transpl 2008;14:779-88. [Crossref] [PubMed]
- Fuks D, Dokmak S, Paradis V, et al. Benefit of initial resection of hepatocellular carcinoma followed by transplantation in case of recurrence: an intention-to-treat analysis. Hepatology 2012;55:132-40. [Crossref] [PubMed]
- Agarwal PD, Lucey MR. Management of hepatocellular carcinoma recurrence after liver transplantation. Ann Hepatol 2022;27:100654. [Crossref] [PubMed]
- Valdivieso A, Bustamante J, Gastaca M, et al. Management of hepatocellular carcinoma recurrence after liver transplantation. Transplant Proc 2010;42:660-2. [Crossref] [PubMed]
- Berardi G, Colasanti M, Ettorre GM. ASO Author Reflections: Pushing the Limits in Laparoscopic Liver Surgery for Hepatocellular Carcinoma. Ann Surg Oncol 2022;29:587-8. [Crossref] [PubMed]
- Au KP, Chok KSH. Immunotherapy after liver transplantation: Where are we now? World J Gastrointest Surg 2021;13:1267-78. [Crossref] [PubMed]
- Zucman-Rossi J, Villanueva A, Nault JC, et al. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015;149:1226-1239.e4. [Crossref] [PubMed]
- Nault JC, De Reyniès A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology 2013;145:176-87. [Crossref] [PubMed]
- Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med 2008;359:1995-2004. [Crossref] [PubMed]
- Pantel K, Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 2010;16:398-406. [Crossref] [PubMed]
- Alix-Panabières C, Pantel K. Liquid biopsy: from discovery to clinical implementation. Mol Oncol 2021;15:1617-21. [Crossref] [PubMed]
- Lei Y, Wang X, Sun H, et al. Association of Preoperative NANOG-Positive Circulating Tumor Cell Levels With Recurrence of Hepatocellular Carcinoma. Front Oncol 2021;11:601668. [Crossref] [PubMed]
- Zhou KQ, Sun YF, Cheng JW, et al. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status in hepatocellular carcinoma. EBioMedicine 2020;62:103107. [Crossref] [PubMed]
- Zhao L, Jiang L, Liu Y, et al. Integrated analysis of circulating tumour cells and circulating tumour DNA to detect minimal residual disease in hepatocellular carcinoma. Clin Transl Med 2022;12:e793. [Crossref] [PubMed]
- Cui K, Ou Y, Shen Y, et al. Clinical value of circulating tumor cells for the diagnosis and prognosis of hepatocellular carcinoma (HCC): A systematic review and meta-analysis. Medicine (Baltimore) 2020;99:e22242. [Crossref] [PubMed]
- Sun C, Liao W, Deng Z, et al. The diagnostic value of assays for circulating tumor cells in hepatocellular carcinoma. Medicine (Baltimore) 2017;96:e7513. [Crossref] [PubMed]
- Fan JL, Yang YF, Yuan CH, et al. Circulating Tumor Cells for Predicting the Prognostic of Patients with Hepatocellular Carcinoma: A Meta Analysis. Cell Physiol Biochem 2015;37:629-40. [Crossref] [PubMed]
- Hao S, Chen S, Tu C, et al. Anterior Approach to Improve the Prognosis in HCC Patients Via Decreasing Dissemination of EpCAM(+) Circulating Tumor Cells. J Gastrointest Surg 2017;21:1112-20. [Crossref] [PubMed]


