
The role of FGFR inhibitors in the treatment of intrahepatic cholangiocarcinoma—unveiling the future challenges in drug therapy
Intrahepatic cholangiocarcinoma (iCCA) is a rare malignancy, constituting approximately 3–5% of hepatic tumors, with an increasing incidence in recent years (1). Early stage iCCA typically presents asymptomatically, often resulting in its detection at advanced stages. Surgical resection remains the primary therapeutic approach for iCCA; however, a substantial proportion of patients are precluded from surgery due to disease progression, necessitating a reliance on pharmaceutical interventions (2).
Currently, the standard therapeutic regimen involves combined administration of gemcitabine and cisplatin (GC) (3). Notably, recent advancements have unveiled a promising regimen, GC combined with durvalumab, an anti-PD-L1 antibody, which has demonstrated efficacy in improving overall survival (OS) and progression-free survival (PFS) compared with GC alone; substantiated by a phase III clinical trial (4). Consequently, the amalgamation of immune checkpoint inhibitors (ICIs) with conventional cytotoxic agents represents a significant advancement in the pharmacotherapeutic landscape of iCCA.
Furthermore, distinctive genetic signatures of cholangiocarcinoma are profoundly influenced by its anatomical localization. iCCAs, which frequently arise within the milieu of chronic liver diseases, harbor mutations in key genes such as fibroblast growth factor receptor 2 (FGFR2), IDH1, IDH2, ARID1A, BAP1, and TP53. In contrast, extrahepatic cholangiocarcinomas commonly manifest with mutations in TP53, KRAS, BRAF, SMAD4, and CDKN2A. Moreover, approximately 12.7% iCCA cases exhibit aberrant FGFR2 (5). This underscores the clinical relevance of FGFR inhibitors in iCCA. They effectively suppress activation of FGF signaling driven by FGFR2 gene fusion or rearrangement, resulting in favorable therapeutic responses. Therefore, the evolving landscape of iCCA management encompasses a continuum of strategies ranging from conventional cytotoxic regimens to molecular-targeted and immunotherapeutic approaches (2). The interplay between advanced pharmaceutical interventions and genetic aberrations hold immense promise for elevating treatment paradigms and propelling the field toward more personalized and efficacious strategies.
Significant efforts have been made to develop pharmacotherapies targeting aberrant FGFR signaling in cholangiocarcinoma. Notably, two adenosine triphosphate (ATP)-competitive reversible FGFR inhibitors, infigratinib and pemigatinib, have been approved by the U.S. Food and Drug Administration (FDA). They have exhibited remarkable clinical promise, as underscored by their respective overall response rates (ORRs) of 23.1% and 35.5% in phase II clinical trials conducted in patients with cholangiocarcinoma with FGFR2 fusions/rearrangements that progressed beyond first-line chemotherapy (6,7).
Furthermore, recent advancements have introduced futibatinib, an irreversible FGFR1-4 inhibitor. Futibatinib covalently binds to cysteine residues within the ATP-binding pocket of FGFR, thereby effectively suppressing FGFR-mediated signaling cascades inducing the inhibition of tumor cell proliferation marked by FGFR1-4 genetic abnormalities, subsequently triggering apoptotic cell death (8).
In the New England Journal of Medicine, published on January 19, 2023, Goyal et al. showed the results of the Phase II FOENIX-CCA2 trial, which evaluated the efficacy and safety of futibatinib monotherapy in patients previously treated FGFR2 fusion/reconstitution-positive iCCA (9). The trial was an open-label single-group study designed to evaluate the ORR of 20 mg futibatinib once daily as the primary endpoint, and the duration of response (DOR), disease control rate (DCR), PFS, and OS as secondary endpoints. Notably, a robust ORR of 42% [95% confidence interval (CI): 32–52%] was reported, accompanied by a median DOR of 9.7 months. Importantly, this favorable response was sustained across a spectrum of patient demographics, including those of advanced age, those with a history of multiple therapeutic interventions, and those bearing TP53 mutations. Over a median follow-up period of 17.1 months, the observed PFS and OS stood at 9.0 and 21.7 months, respectively.
The therapeutic landscape of futibatinib is characterized by a manageable safety profile. Notable grade 3 treatment-related adverse events (TRAEs) encompassed hyperphosphatemia, elevated aspartate aminotransferase levels, stomatitis, and fatigue, with incidences of 30%, 7%, 6%, and 6%, respectively. Treatment discontinuation stemming from TRAEs occurred in only 2% of patients, with no treatment-related deaths. Based on these results, the U.S. FDA accepted a new drug application for futibatinib with priority review designation in March 2022. This development is a pivotal step towards expanding the therapeutic armamentarium available for the management of FGFR2-driven cholangiocarcinoma and fuels aspiration for enhanced patient outcomes.
In the context of novel therapeutic agents, futibatinib has emerged as a distinct entity from conventional FGFR inhibitors, prompting a critical clinical investigation into its potential efficacy against FGFR resistant tumors rendering them unresponsive to reversible ATP-competitive FGFR inhibitors (10). FGFRs can be categorized into four distinct types: FGFR1 through FGFR4. Notably, futibatinib acts by irreversibly suppressing the activity of all FGFR1-4 variants, irrespective of the nuanced structural differences inherent to FGFR kinases. However, a pertinent caveat prevails: The FOENIX-CCA2 trial deliberately excluded patients with prior exposure to FGFR inhibitors, thereby raising concerns about the clinical efficacy of futibatinib with respect to conventional FGFR inhibitor resistant tumors (9).
A pertinent preclinical investigation, by Sootome et al., aimed to elucidate the behavior of futibatinib against clones harboring reversible ATP-competitive inhibitor resistant mutations. In this study, FGFR2 inhibitors were administered to cell lines derived from the human fetal kidney (HEK293T cells) expressing both wild-type and mutant FGFR2 (8). A discernible attenuation in the inhibitory potential was observed within cells expressing mutant FGFR2 upon exposure to conventional reversible ATP-competitive inhibitors. Notably, futibatinib exhibited an unyielding and sustained FGFR-inhibitory effect, even when confronted with the mutant clone.
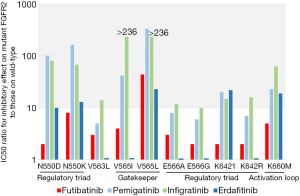
In a concerted effort to deepen our understanding, the same group sought to engineer somatic mutations via random mutagenesis in mouse pre-B-cell-derived cell lines treated with various FGFR inhibitors (11). Sequencing of the kinase domain FGFR2 in FGFR inhibitor resistant clones yielded informative results (Figure 1). While a multitude of mutations have emerged in the regulatory triad, gatekeeper, and activation loop regions of the kinase domain among reversible ATP-competitive FGFR inhibitor resistant clones, a solitary mutation has emerged as a sentinel to irreversible FGFR1-4 inhibitor resistance; V565L mutation located in the gatekeeper region of the FGFR2 kinase domain. This comprehensive investigation provides valuable insights into the mechanisms of resistance and underscores the distinctiveness of futibatinib’s mode of action, offering a promising vantage point for further clinical exploration.
Interestingly, a case of human iCCA refractory to pemigatinib has been reported, wherein futibatinib successfully demonstrated an anti-tumor effect (12). The N549D mutation within the FGFR2 gene was identified in tumors refractory to pemigatinib. Remarkably, the application of futibatinib elicited tumor regression, accompanied by a reduction in CA19-9 levels, without the resurgence of N549D on liquid biopsy. This instance highlights the potential significance of the vigilant monitoring of FGFR2 mutation profiles during FGFR inhibitor therapy, an avenue that can be explored through the analysis of both tumor biopsy specimens and peripheral blood samples.
Acquisition of tumor tissue via biopsy offers direct access to tumor material, enabling precise mutation analysis and histological assessment, including immunostaining for tumor immune microenvironment (TIME) profiling, critical for the rational deployment of ICIs. Nonetheless, tumor biopsies may pose undue invasiveness and hinge on the accessibility of the target lesion. Additionally, the inherent heterogeneity of tumor mutations within minute biopsy specimens may limit the detection of emerging drug-resistant clones. In contrast, liquid biopsy, based on the analysis of tumor-released DNA within the bloodstream, is a less invasive and potentially repetitive modality. Comprehensive DNA interrogation spanning the entire tumor milieu attenuates the impact of heterogeneity-related detection biases. However, optimal DNA yield and sensitivity for minor variants are pivotal requirements along with the need to ascertain the precise origin of the detected variants, all of which pose challenges to the clinical utility of liquid biopsies. More importantly, the underlying mechanisms governing resistance to FGFR inhibitors may extend beyond mutations in the FGFR kinase domain. Therefore, further investigation is required to determine the utility of monitoring FGFR2 mutation profiles during treatment with FGFR inhibitors.
Conversely, the combination of an anti-PD-L1 antibody and GC has been approved for iCCA cases; however, its efficacy in patients refractory to FGFR inhibitors remains unknown (4). Notably, ICIs efficacy is intrinsically linked to the intricate TIME. A prevailing trend associates FGFR2 fusion/rearrangement cases, which constitute prime candidates for FGFR inhibitors, with a “non-inflamed” classification characterized by limited T-cell infiltration within the tumor (13). Consequently, the therapeutic effects of ICIs may be blunted in patients eligible for treatment with FGFR inhibitors. However, an alternative perspective delineates FGFR2 alterations as instigators of FGF signaling activation, leading to the induction of the suppressor of cytokine signaling 1, a suppressor of interferon-γ signaling (14). This, in turn, hampers the upregulation of human leukocyte antigen (HLA) through the interferon-γ pathway, potentially perpetuating the “non-inflamed” tumor state (Figure 2). Herein lies a provocative proposition: FGFR inhibitors may recalibrate the suppressive TIME by promoting HLA induction, thus presenting a rationale for combining FGFR inhibitors with ICIs and forging a new therapeutic avenue for iCCA (15).

Our previous findings also highlighted the promise of IDH1 inhibitors for rectifying the immune milieu and amplifying the intrigue of multifaceted therapeutic strategies (15). In summary, the therapeutic landscape for refractory iCCA is swiftly evolving, featuring a comprehensive armamentarium of FGFR inhibitors, including novel agents, such as futibatinib, IDH1 inhibitors, and ICIs. Although the development of novel drug therapeutic strategies for refractory iCCA is challenging, the field is poised for rapid progression and is underscored by an expansive repertoire of ongoing clinical trials (2).
Acknowledgments
Funding: This work was supported in part by a Grant-in-Aid for Scientific Research from
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-411/coif). The author reports that this work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI: 21K07184), and a grant from Smoking Research Foundation.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology 2013;145:1215-29. [Crossref] [PubMed]
- Ilyas SI, Affo S, Goyal L, et al. Cholangiocarcinoma - novel biological insights and therapeutic strategies. Nat Rev Clin Oncol 2023;20:470-86. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Oh D-Y, He AR, Qin S, et al. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid 2022;1.
- Lowery MA, Ptashkin R, Jordan E, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res 2018;24:4154-61. [Crossref] [PubMed]
- Abou-Alfa GK, Sahai V, Hollebecque A, et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol 2020;21:671-84. [Crossref] [PubMed]
- Javle M, Roychowdhury S, Kelley RK, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol 2021;6:803-15. [Crossref] [PubMed]
- Sootome H, Fujita H, Ito K, et al. Futibatinib Is a Novel Irreversible FGFR 1-4 Inhibitor That Shows Selective Antitumor Activity against FGFR-Deregulated Tumors. Cancer Res 2020;80:4986-97. [Crossref] [PubMed]
- Goyal L, Meric-Bernstam F, Hollebecque A, et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N Engl J Med 2023;388:228-39. [Crossref] [PubMed]
- Rizzo A, Ricci AD, Brandi G. Futibatinib, an investigational agent for the treatment of intrahepatic cholangiocarcinoma: evidence to date and future perspectives. Expert Opin Investig Drugs 2021;30:317-24. [Crossref] [PubMed]
- Sootome H, Kato S, Kato M, et al. Acquired resistance to ATP-competitive and irreversible FGFR inhibitors (FGFRi's): A library-based approach. Cancer Res 2021;81:1117. abstract. [Crossref]
- Rengan AK, Denlinger CS. Robust Response to Futibatinib in a Patient With Metastatic FGFR-Addicted Cholangiocarcinoma Previously Treated Using Pemigatinib. J Natl Compr Canc Netw 2022;20:430-5. [Crossref] [PubMed]
- Martin-Serrano MA, Kepecs B, Torres-Martin M, et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut 2023;72:736-48. [Crossref] [PubMed]
- Adachi Y, Kamiyama H, Ichikawa K, et al. Inhibition of FGFR Reactivates IFNgamma Signaling in Tumor Cells to Enhance the Combined Antitumor Activity of Lenvatinib with Anti-PD-1 Antibodies. Cancer Res 2022;82:292-306. [Crossref] [PubMed]
- Nishida N, Aoki T, Morita M, et al. Non-Inflamed Tumor Microenvironment and Methylation/Downregulation of Antigen-Presenting Machineries in Cholangiocarcinoma. Cancers (Basel) 2023;15:2379. [Crossref] [PubMed]


