
Fibroscan-AST (FAST) score and other non-invasive tests for the diagnosis of fibrotic non-alcoholic steatohepatitis
The Fibroscan-AST (FAST) score was developed based on the data of patients who had a liver biopsy for non-alcoholic fatty liver disease (NAFLD) at several liver centres in England, and it was validated using data from seven clinical studies from North America, Europe and Asia (1). The FAST score requires only controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) from a vibration-controlled transient elastography (VCTE) examination and serum aspartate aminotransferase (AST) level in its calculation. While the formula is available publicly, a free application called myFibroScan (Echosens, Paris, France) can be used to easily calculate the FAST score (2), as well as two other VCTE-based scores called Agile 3+ and Agile 4 (3). The diagnostic goal for the FAST score is fibrotic non-alcoholic steatohepatitis (NASH), which is defined histologically as the presence of NASH with a NAFLD activity score of ≥4 and significant fibrosis (≥F2). Fibrotic NASH has been identified as the target for clinical trials of emerging pharmacotherapies for NAFLD, an area which has seen an explosion of activities in recent years due to the significant unmet need.
In a recently published systematic review and meta-analysis (4), Ravaioli and colleagues included twelve studies with a total of 5,835 patients who underwent a liver biopsy for NAFLD, and reported that the FAST score had an area under the receiver operating characteristic curve (AUROC) of 0.79, with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 89%, 89%, 65% and 92%, respectively, for the diagnosis of fibrotic NASH, with 33% of patients falling within the intermediate zone using the ≤0.35 and ≥0.67 cut-offs from the original study (1). The proportion of patients with fibrotic NASH was 28% in the study population. These values are remarkably similar to those from the external validation cohort of the original study, lending support to the robustness of the original study. When using the FAST score, as in any diagnostic tests, we should remember that the predictive values can be affected by the prevalence of the diagnostic goal in the tested population. In the primary care setting, where the prevalence of fibrotic NASH is much lower, the FAST score will have lower PPV, although its NPV will remain good. When using the FAST score as a pre-screening tool for clinical trials, it is also important to consider the trade-off between screen failure rate and missed case rate. For example, if only patients with FAST score ≥0.67 were selected to undergo screening, although the screen failure rate decreased from 72% to 31%, it was associated with a missed case rate of 52%. On the other hand, using the >0.35 cut-off decreased the missed case rate to 11%, but increased the screen failure rate to 51%. We should also be mindful that the diagnostic goal of fibrotic NASH includes patients with cirrhosis. If the FAST score was used as a pre-screening tool for a clinical trial that enrols only patients with NASH and F2 or F3 fibrosis, additional screen failures can be expected from patients with cirrhosis.
There are a few other non-invasive tests developed specifically for the diagnosis of fibrotic NASH (see Table 1). MACK-3 incorporates homeostasis model assessment of insulin resistance, serum AST and cytokeratin-18 levels to diagnose fibrotic NASH, showing a diagnostic accuracy of 93.2% and 79.1% in the original study and in an external validation study, respectively (5,6). Another score called the NIS4 panel, which utilizes microRNA-34a-5p, alpha-2-macroglobulin, YKL-40 and hemoglobin A1c, demonstrated an AUROC of 0.76 to 0.83 for the diagnosis of fibrotic NASH (7). Recently, Govaere and colleagues eloquently detailed proteo-transcriptomic map of NASH and hepatic fibrosis during progressive NAFLD. From a total of 31 signature proteins identified in different hepatic cell populations after integrating proteomics (using the proteomic aptamer-based SomaScan Platform) and RNA sequencing approaches, a classification model was developed to diagnose fibrotic NASH using four exemplary circulating proteins (ADAMTSL2, AKR1B10, CFHR4 and TREM2), body mass index and type 2 diabetes mellitus status. This composite model identified fibrotic NASH with an AUROC of 0.88 in the discovery cohort and had an AUROC of 0.80 in the validation cohort (8). A comparative diagnostic accuracy study from the Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS) project showed that the proteomic based SomaSignal test (a modified, aptamer-based logistic regression model) demonstrated the best performance for the diagnosis of fibrotic NASH with an AUROC of 0.81 (10). Noureddin and colleagues developed the MRI-AST (MAST) score, which combines steatosis measured with MRI-based proton density fat fraction (MRI-PDFF), liver stiffness measured with MR elastography (MRE), and serum AST level, for the diagnosis of fibrotic NASH. The MAST score had an impressive AUROC of 0.93, which was superior to the FAST score (9).
Table 1
| Test | Prevalence of fibrotic NASH | AUROC (95% CI) |
Cut-offs | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|---|---|---|
| MACK-3 (HOMA-IR, AST, CK-18): Original study (5) | 23.3% | 0.85±0.02 | ≤0.134 (rule-out fibrotic NASH) | 90% at cut-off ≤0.134 |
94.2% at cut-off ≥0.550 |
83.4 | 96.9 |
| ≥0.550 (rule-in fibrotic NASH) | |||||||
| MACK-3: External validation cohort (6) | 21.4% | 0.80 (0.74–0.87) | ≤0.134 (rule-out fibrotic NASH) | 100% at cut-off ≤0.134 |
43.8% at cut-off ≥0.550 |
43.1 | 100 |
| ≥0.550 (rule-in fibrotic NASH) | |||||||
| NIS4 algorithm (miR-34a-5p, alpha-2-macroglobulin, YKL-40, and HbA1C) (7) | 44–49% | 0.80 (0.77–0.84) | <0.36 to rule-out fibrotic NASH; | 81.5% (76.9–85.3) at <0.36 cutoff |
87.1% (83.1–90.3) at ≥0.63 cutoff |
79.2 (73.1–84.2) |
77.9 (72.5–82.4) |
| ≥0.63 to rule-in fibrotic NASH | |||||||
| Proteomic-based classification model (4 circulating proteins, BMI, HbA1C) | NA | Discovery cohort: 0.88 (±0.03) | >−0.4491 to rule-in fibrotic NASH | NA | NA | 79.0 | 85.0 |
| Validation cohort: 0.80 (±0.04) | <−0.4491 to rule-out fibrotic NASH | ||||||
| SomaSignal test (composed of 35 different proteins) (8) | 46% | 0.81 (0.75–0.86) | Threshold 0.06 to rule-in fibrotic NASH | 67.0% (59.0–75.0) |
82.0% (59.0–75.0) |
NA | NA |
| MAST score (9) | Derivation cohort: 17.5% | 0.86 (0.78–0.93) | <0.165 rule-out fibrotic NASH | 94.4% | 72.9% | 42.5 | 98.4 |
| >0.242 rule-in fibrotic NASH | 61.1% | 89.4% | 55.0 | 91.6 | |||
| Validation cohort: 11.5% | 0.93 (0.88–0.97) | <0.165 rule-out fibrotic NASH | 89.3% | 72.2% | 29.4 | 98.1 | |
| >0.242 rule-in fibrotic NASH | 75.0% | 90.3% | 50.0 | 96.5 |
NASH, non-alcoholic steatohepatitis; AUROC, areas under the receiver operating characteristic curves; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value; HOMA-IR, homeostasis model assessment for insulin resistance; AST, aspartate aminotransferase; CK-18, cytokeratin-18 fragments; BMI, body mass index; MAST, MRI-AST score; HbA1C, glycosylated hemoglobin; NA, not available.
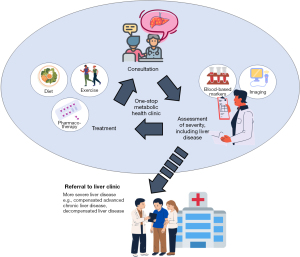
The choice of test used for the diagnosis of fibrotic NASH depends not only on its accuracy, but also on cost and availability. The blood-based scores mentioned above requires markers that are not routinely available and/or the use of a proprietary algorithm. On the other hand, MRI-based tests, especially MRE, are not readily available and costly. Furthermore, MRI-based tests are not point-of-care tests, unlike VCTE. Besides the FAST score, other VCTE-based scores, namely Agile 3+ and Agile 4, which use readily available parameters in addition to LSM, can reduce the proportion of patients with indeterminate results and improve the PPVs compared with LSM alone for the diagnosis of advanced fibrosis and cirrhosis, respectively (3). Currently, the American Association for the Study of Liver Diseases and the European Association for the Study of Liver both recommends the use of sequential testing, namely fibrosis-4 score as the initial test, followed by a second test (e.g., LSM, using the 8 and 12 kPa cut-offs), for the identification of patients with advanced fibrosis (11,12). The 6th Edition of the Clinical Practise Guidelines on the Management of Type 2 Diabetes Mellitus in Malaysia and the Malaysian Society of Gastroenterology and Hepatology consensus statements on metabolic dysfunction associated fatty liver disease (MAFLD) provides a similar recommendation albeit using higher cut-offs for LSM at 10 and 15 kPa (13,14). There is no clear recommendation on the use of the VCTE-based scores or the other non-invasive scores targeting fibrotic NASH in the guidelines. While data on non-invasive tests as an alternative to histology for the prediction of long-term clinical outcomes are emerging (e.g., for LSM) (15), more work needs to be done for VCTE-based scores and the other non-invasive scores targeting fibrotic NASH in this aspect. Further studies and guidance are also needed on the use of non-invasive tests for the selection of patients for pharmacotherapy when this becomes available, and to monitor their response to treatment. A simple, accurate and affordable test (or combination of tests) that can be used to diagnose and provide prognostic information (which can be used to guide decision for referral to the liver clinic) and to guide treatment decision and monitor response is much needed (see Figure 1).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-346/coif). WKC received consulting fees from Roche, Abbvie, Boehringer Ingelheim and Novo Nordisk, payment for lectures from Echosens, Viatris, Hisky Medical and Novo Nordisk, and travel grant from Novo Nordisk. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:362-73. [Crossref] [PubMed]
- Echosens. myFibroScan. 2023 9 July 2023). Available online: https://www.echosens.com/products/my-fibroscan/
- Sanyal AJ, Foucquier J, Younossi ZM, et al. Enhanced diagnosis of advanced fibrosis and cirrhosis in individuals with NAFLD using FibroScan-based Agile scores. J Hepatol 2023;78:247-59. [Crossref] [PubMed]
- Ravaioli F, Dajti E, Mantovani A, et al. Diagnostic accuracy of FibroScan-AST (FAST) score for the non-invasive identification of patients with fibrotic non-alcoholic steatohepatitis: a systematic review and meta-analysis. Gut 2023;72:1399-409. [Crossref] [PubMed]
- Boursier J, Anty R, Vonghia L, et al. Screening for therapeutic trials and treatment indication in clinical practice: MACK-3, a new blood test for the diagnosis of fibrotic NASH. Aliment Pharmacol Ther 2018;47:1387-96. [Crossref] [PubMed]
- Chuah KH, Wan Yusoff WNI, Sthaneshwar P, et al. MACK-3 (combination of hoMa, Ast and CK18): A promising novel biomarker for fibrotic non-alcoholic steatohepatitis. Liver Int 2019;39:1315-24. [Crossref] [PubMed]
- Harrison SA, Ratziu V, Boursier J, et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol 2020;5:970-85. [Crossref] [PubMed]
- Govaere O, Hasoon M, Alexander L, et al. A proteo-transcriptomic map of non-alcoholic fatty liver disease signatures. Nat Metab 2023;5:572-8. [Crossref] [PubMed]
- Noureddin M, Truong E, Gornbein JA, et al. MRI-based (MAST) score accurately identifies patients with NASH and significant fibrosis. J Hepatol 2022;76:781-7. [Crossref] [PubMed]
- Vali Y, Lee J, Boursier J, et al. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol 2023;8:714-25. [Crossref] [PubMed]
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797-835. [Crossref] [PubMed]
- European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel; Chair:; EASL Governing Board representative:; Panel members:. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol 2021;75:659-89.
- Chan WK, Tan SS, Chan SP, et al. Malaysian Society of Gastroenterology and Hepatology consensus statement on metabolic dysfunction-associated fatty liver disease. J Gastroenterol Hepatol 2022;37:795-811. [Crossref] [PubMed]
- Clinical Practise Guidelines Management of Type 2 Diabetes Mellitus (6th Edition). 2020, Malaysia Endocrine & Metabolic Society, Ministry of Health Malaysia, Academy of Medicine Malaysia, Diabetes Malaysia and Family Medicine Specialists Association of Malaysia: Putrajaya.
- Mózes FE, Lee JA, Vali Y, et al. Performance of non-invasive tests and histology for the prediction of clinical outcomes in patients with non-alcoholic fatty liver disease: an individual participant data meta-analysis. Lancet Gastroenterol Hepatol 2023;8:704-13. [Crossref] [PubMed]


