
Impact of low alcohol consumption in the natural history of cirrhosis
Alcohol-related liver disease (ALD) and metabolic dysfunction-associated steatotic liver disease (MASLD) are the most common causes of chronic liver disease worldwide (1). Daily alcohol consumption thresholds (<20 g/day for women and <30 g/day for men) are used to arbitrarily differentiate MASLD from ALD (2). However, “safe” levels of alcohol intake are difficult to define because of wide variations in the factors that contribute to its susceptibility and multiple effect modifiers. In MASLD, conflicting results on whether light alcohol intake is detrimental or beneficial, add uncertainty to hepatologists as to recommend tight abstinence or allow low levels of alcohol use (3). There is also little evidence regarding alcohol exposure once hepatic cirrhosis has developed and the impact of different thresholds in its natural history. These knowledge gaps are currently under intense debate.
Information about the long-term outcomes driven by drinking behaviors in patients with cirrhosis of any etiology is of great relevance. Two studies from prospective data registries, one in MASLD and the other in ALD have tried to fill this research gap (4,5). Table 1 summarizes the main differences between both studies. In MASLD cirrhosis, after a mean follow-up period of 5 years, liver transplantation (LT) and mortality rate were both 8% (4). Importantly, alcohol consumption (defined as 1–70 grams/week for women and 1–140 grams/week for men) was associated with a higher risk of mortality and liver decompensation (4). Given that up to 25% of patients categorized as MASLD can underreport excessive alcohol consumption, it is not surprising that further epidemiological studies have also suggested that the increased mortality among MASLD can be driven by ALD (6,7).
Table 1
| Study data | MASLD cirrhosis (4) | ALD cirrhosis (5) |
|---|---|---|
| Countries/regions | Spain, Australia, Hong Kong, Cuba | France, Belgium |
| Time period | Inclusion: 1995–2013 | Inclusion: 2010–2016 |
| Follow-up: median, 66 (range, 32–98) months | Follow-up: median, 46 (range, 33–70) months | |
| Population | n=458 biopsy-proven F3–4 MASLD; 65% compensated cirrhosis | n=650 biopsy-proven ALD; 100% compensated cirrhosis |
| Cirrhosis stage | 74% CTP-A5, MELD 7.7; no history of decompensation | 82% CTP-A5, MELD 8.6; 64% history of decompensation |
| Comorbidities | Age: 56 years; male: 48%; BMI: 33 kg/m2; diabetes: 67%; tobacco: 30% | Age: 58 years; male: 67%; BMI: 28 kg/m2; diabetes: 23%; tobacco: 76% |
| Alcohol status | >1 glass/week: 14%; abstinent at baseline: 86%; abstinent during follow-up: 88%; new onset drinkers: 0% | >1 glass/week: 30%; abstinent: 70%; abstinent during follow-up: 51%; new onset drinkers: 19% |
| Clinical events | Hepatic decompensation, HCC: 19%, 9%; liver transplantation: 8%; all-cause mortality: 8%: non-liver: 16% (mostly CVE), liver-related: 84% | Hepatic decompensation, HCC: 15%, 9%; liver transplantation: 0.5%; all-cause mortality: 24%: non-liver: 46% (mostly cancer), liver-related: 54% |
MASLD, metabolic dysfunction-associated steatotic liver disease; ALD, alcohol-related liver disease; MELD, Model for End-Stage Liver Disease; BMI, body mass index; CTP, Child-Turcotte-Pugh; HCC, hepatocellular carcinoma; CVE, cardiovascular event.
On the other hand, in ALD cirrhosis, data was lacking until Louvet and collaborators recently published their study based on the CIRRAL cohort (5). Several key clinical implications must be highlighted in this French multicenter study:
- Recurrence of alcohol intake in abstinent patients is twice more prevalent than alcohol withdrawal in non-abstinent patients: 30% vs. 14% after 5 years of ALD cirrhosis diagnosis. A shorter duration of alcohol discontinuation was a predictive factor of recurrence. In line with this finding, recent sobriety has also been associated with higher rates of incident alcohol withdrawal syndrome in patients with alcohol-associated hepatitis (8).
- A past medical history of liver decompensation is not associated with the probability of recurrence of alcohol intake. As mentioned by the authors, this is relevant in the field of ALD as the “general consensus” is to not consider a patient for LT in case of previous decompensated cirrhosis. Such “general consensus” is illustrated in Table 1 as patients with ALD cirrhosis (64% with previous decompensation) had far less access to LT than MASLD cirrhosis (0% with previous decompensation): 0.5% in ALD vs. 8% in MASLD.
- Overall mortality was high (24%) and with a similar distribution between liver and non-liver-related causes of death. Very similar cause-specific mortality in ALD has been recently shown in a population-based study from Denmark, highlighting the risk of cancer other than hepatocellular carcinoma (HCC) (9). Importantly, the risk of all-cause mortality in ALD was three times higher compared to MASLD, indicating a very different prognosis according to cirrhosis etiology (Table 1).
- Independent predictors of outcomes. In ALD cirrhosis, the risk of either death or liver complications was higher in non-abstinent patients, increasing in a dose-dependent manner from a low consumption level (1–6 glasses/week) to the greatest consumption level (≥28 glasses/week). These results have been recently corroborated in an independent Greek cohort (10). Thus, low-to-moderate alcohol consumption (1–7 glasses/week for women and 1–14 glasses/week for men) might likely increase the likelihood of adverse outcomes in ALD and MASLD cirrhotic patients (4,5). Of note, Louvet et al. also confirmed that higher consumption of coffee was associated with a 40% decrease in overall mortality or liver-related events (5). Conversely, in MASLD cirrhosis, cigarette smoking was associated with a 75% increase in overall mortality and HCC (4,11).
- To assess the role of metabolic conditions in predicting outcomes, overall survival was also evaluated in ALD patients without metabolic syndrome. In these patients with lower metabolic risk, although similar results were obtained, the impact of alcohol consumption on mortality remained only statistically significant for the greatest levels (≥28 glasses/week). These results can reflect a supra-additive effect between metabolic syndrome and alcohol consumption, where even low intake might likely influence outcomes (1).
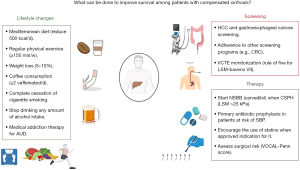
In summary, these new findings may assist clinicians in managing patients with cirrhosis. Figure 1 summarizes what can be easily done to improve survival among patients with compensated cirrhosis (11-15). Given that outcomes are worse among non-abstinent patients with ALD or MASLD cirrhosis, all patients should be advised to completely stop drinking, and hepatologists and addiction specialists should work together to ensure long-term abstinence. No level of alcohol consumption can be regarded as safe when cirrhosis is diagnosed. More research is needed to clarify the detrimental effects of light to moderate alcohol consumption on the risk of death and adverse liver outcomes among non-cirrhotic patients with or without major metabolic comorbidities.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-615/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Åberg F, Byrne CD, Pirola CJ, et al. Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease. J Hepatol 2023;78:191-206. [Crossref] [PubMed]
- Rinella ME, Lazarus JV, Ratziu V, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023;78:1966-86. [Crossref] [PubMed]
- Díaz LA, Arab JP, Louvet A, et al. The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol 2023;20:764-83. [Crossref] [PubMed]
- Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018;155:443-457.e17. [Crossref] [PubMed]
- Louvet A, Bourcier V, Archambeaud I, et al. Low alcohol consumption influences outcomes in individuals with alcohol-related compensated cirrhosis in a French multicenter cohort. J Hepatol 2023;78:501-12. [Crossref] [PubMed]
- Staufer K, Huber-Schönauer U, Strebinger G, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol 2022;77:918-30. [Crossref] [PubMed]
- Younossi ZM, Paik JM, Al Shabeeb R, et al. Are there outcome differences between NAFLD and metabolic-associated fatty liver disease? Hepatology 2022;76:1423-37. [Crossref] [PubMed]
- Marti-Aguado D, Gougol A, Gomez-Medina C, et al. Prevalence and clinical impact of alcohol withdrawal syndrome in alcohol-associated hepatitis and the potential role of prophylaxis: a multinational, retrospective cohort study. EClinicalMedicine 2023;61:102046. [Crossref] [PubMed]
- Kann AE, Jepsen P, Madsen LG, et al. Cause-specific mortality in patients with alcohol-related liver disease in Denmark: a population-based study. Lancet Gastroenterol Hepatol 2023;8:1028-34. [Crossref] [PubMed]
- Kalambokis GN, Chouliara N, Tsiakas I, et al. Impact of continued alcohol use on liver-related outcomes of alcohol-associated cirrhosis: a retrospective study of 440 patients. Eur J Gastroenterol Hepatol 2024;36:89-96. [Crossref] [PubMed]
- Marti-Aguado D, Clemente-Sanchez A, Bataller R. Cigarette smoking and liver diseases. J Hepatol 2022;77:191-205. [Crossref] [PubMed]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367-78.e5; quiz e14-5. [Crossref] [PubMed]
- Tapper EB, Parikh ND. Diagnosis and Management of Cirrhosis and Its Complications: A Review. JAMA 2023;329:1589-602. [Crossref] [PubMed]
- de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022;76:959-74. [Crossref] [PubMed]
- Vannier AGL, Shay JES, Fomin V, et al. Incidence and Progression of Alcohol-Associated Liver Disease After Medical Therapy for Alcohol Use Disorder. JAMA Netw Open 2022;5:e2213014. [Crossref] [PubMed]


