
Heterogeneity in the risk of incident liver cirrhosis driven by PNPLA3 genotype and diabetes among different populations
Advanced fibrosis determines the prognosis of nonalcoholic fatty liver disease (NAFLD); therefore, accurate risk-prediction for advanced fibrosis is fundamental in the management of NAFLD (1). Targeted screening for advanced fibrosis in high-risk populations, such as those with type 2 diabetes, obesity with metabolic complications, a family history of cirrhosis, or significant alcohol use is recommended for implementing early intervention to prevent future adverse hepatic outcomes of NAFLD (2,3). Non-invasive tests including fibrosis-4 index (FIB-4) and liver stiffness measurement (LSM) are recommended to identify individuals at a higher risk of liver-related events (1,2). In addition to clinical classifications, such as FIB-4; genetic information provides further stratification for liver-related outcomes (4).
Recently, Chen et al. reported that the PNPLA3-rs738409 genotypes and diabetes status identified patients at a higher risk of cirrhosis among those already at an indeterminate risk of NAFLD (FIB-4: 1.3–2.67) in two independent cohorts involving the Michigan Genomics Initiative (MGI) and United Kingdom Biobank (UKBB). NAFLD patients with FIB-4: 1.3–2.67, in the presence of PNPLA3-rs738409 GG genotype and diabetes had a cirrhosis incidence-risk comparable to those with FIB-4 >2.67 (5).
Since the participants from MGI and UKBB were mainly Caucasians; we investigated whether the occurrence of diabetes and PNPLA3-rs738409 GG genotype identified at-risk population with NAFLD carrying an intermediate risk using an Asian biopsy-proven NAFLD cohort (6). Based on serial assessments of LSM by transient elastography, at least 1 year apart (n=267), fibrosis progression was defined as a composite of (I) LSM ≥9.6 kPa during the follow-up period for patients with F0–2 at baseline (7), and (II) ΔLSM ≥+20% compared to the baseline level during the follow-up period for patients with F3–4 at baseline (8). According to baseline FIB-4 values (<1.3, 1.3–2.67, >2.67), diabetes status, and PNPLA3 genotype, the risk of fibrosis progression was assessed using the Cox proportional hazards model. Compared to those with FIB-4 <1.3 at baseline and CC + CG genotype, individuals with FIB-4 1.3–2.67, diabetes, and GG genotype showed 8.52 times higher risk of fibrosis progression [95% confidence interval (CI): 2.13–34.08; Figure 1], which was not statistically different from that of individuals with FIB-4 >2.67 (P=0.550), corresponding to the result by Chen et al.
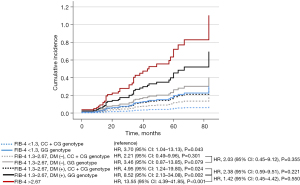
Chen et al. suggested that NAFLD with FIB-4: 1.3–2.67, diabetes, and GG genotype could be categorized as the high-risk group (5). However, the difference in the effect of PNPLA3 genotype on the risk of cirrhosis and the difference in the absolute incidence-rate of cirrhosis between individuals from MGI and UKBB, should be carefully interpreted to apply the risk-stratifying strategy in a clinical setting.
MGI and UKBB are prospective cohorts based on the tertiary care center and community, respectively (5). Due to this fundamental difference in participants from MGI and UKBB, there were marked differences in the prevalence of cardiometabolic risk factors between the cohorts (5). The prevalence of diabetes and obesity was higher in MGI compared to that of UKBB (diabetes: 35.5% vs. 9.7%; obesity: 57.0% vs. 46.2%). Moreover, the prevalence of class III obesity and coronary heart disease in MGI reached 14.9% and 20.2%, respectively, which were agreeably higher than 4.0% and 8.8% in UKBB (5). Despite the lack of data on the sensitivity of ICD-9/10 code-based definition of cirrhosis in each cohort, the difference in cirrhosis-incidence between MGI and UKBB during the follow-up period may correspond to the difference in the cardiometabolic risk found in these cohorts at baseline. The incidence of cirrhosis was 4.0 per 1,000 person-years (PY) (median follow-up, 71.6 months) and 0.6 per 1,000 PY (median follow-up, 106.3 months) in MGI and UKBB, respectively (5). In MGI, even those with FIB-4 <1.3, no diabetes, and the presence of GG genotype showed a higher incidence-rate of cirrhosis (7.68 per 1,000 PY) than patients with NAFLD from UKBB with FIB-4: 1.3–2.67, diabetes, and GG genotype (3.72 per 1,000 PY). As suggested by Chen et al., NAFLD with FIB-4: 1.3–2.67, diabetes, and GG genotype may be categorized as the high-risk group; however, individuals satisfying these criteria in UKBB showed a lower incidence of cirrhosis than those with NAFLD and FIB-4 <1.3 from the hospital-based cohort. In MGI, the PNPLA3 genotype can identify those at a high risk of incident cirrhosis in individuals with FIB-4 <1.3 and no diabetes (CC + CG, 1.93 per 1,000 PY; GG, 7.68 per 1,000 PY). Moreover, the effect of PNPLA3 genotype on cirrhosis-incidence was more pronounced in individuals without diabetes as opposed to those with diabetes [incidence rate ratio, 3.98 (95% CI: 3.00–5.27) vs. 0.80 (95% CI: 0.10–6.35)].
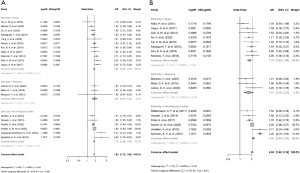
The heterogeneity in the effect of PNPLA3 genotype on NAFLD progression should be also considered in the development of risk-stratifying model incorporating genotypes. Using PubMed and Embase, we searched for studies investigating the association between the PNPLA3 genotype and NAFLD severity. Inclusion criteria were studies with (I) liver biopsy or magnetic resonance imaging to define NAFLD or nonalcoholic steatohepatitis (NASH), (II) PNPLA3 rs738409 genotype, and (III) information on the ethnicity of study subjects. Exclusion criteria were studies with (I) study subjects <100, (II) absence of ethnicity data, (III) no genotype-matched outcomes, or (IV) abstracts or posters only. Among the 1,044 articles identified through database searching, 20 studies were included in the analysis. Overall, the risk of NAFLD increased 1.80 times (95% CI: 1.72–1.88) per 1G allele (Figure 2A). There was no ethnic difference in the effect of PNPLA3 genotype and NAFLD (P=0.960). Instead, significant heterogeneity among the studies in each ethnic group as well as in the entire studies was found (P=0.018 and 0.030, respectively). The association between PNPLA3 genotype and NASH in NAFLD population based on ethnicity was also analyzed, and statistically significant heterogeneity was found (P<0.001; Figure 2B).
It must be acknowledged that diabetes and PNPLA3 genotype are important factors for determination of liver cirrhosis in NAFLD. However, the absolute risk associated with the presence of risk factors in NAFLD patients may vary across the study populations. The expert guidelines recommend that the approach for evaluating the risk of advanced NAFLD should be based on the prevalence of advanced disease in each population (2). As compared to hepatology practices, the objective of risk assessment in a primary healthcare setting is to identify patients who are unlikely to have advanced fibrosis as the prevalence of advanced NAFLD may be relatively low (2,3). To establish the risk-stratifying strategy, the prevalence of advanced liver disease and co-morbidities associated with NAFLD severity in the target population should be considered (9). When predicting the risk, particularly through the use of risk genotypes, it is essential to consider the heterogeneity in the effects of genotypes within each population.
Acknowledgments
Funding: This study was supported by
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-624/coif). The authors report that this study was supported by Korea National Institute of Health (2022ER090700). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Stern C, Castera L. Identification of high-risk subjects in nonalcoholic fatty liver disease. Clin Mol Hepatol 2023;29:S196-206. [Crossref] [PubMed]
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797-835. [Crossref] [PubMed]
- Kanwal F, Shubrook JH, Adams LA, et al. Clinical Care Pathway for the Risk Stratification and Management of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2021;161:1657-69. [Crossref] [PubMed]
- De Vincentis A, Tavaglione F, Jamialahmadi O, et al. A Polygenic Risk Score to Refine Risk Stratification and Prediction for Severe Liver Disease by Clinical Fibrosis Scores. Clin Gastroenterol Hepatol 2022;20:658-73. [Crossref] [PubMed]
- Chen VL, Oliveri A, Miller MJ, et al. PNPLA3 Genotype and Diabetes Identify Patients With Nonalcoholic Fatty Liver Disease at High Risk of Incident Cirrhosis. Gastroenterology 2023;164:966-977.e17. [Crossref] [PubMed]
- Koo BK, Lee H, Kwak SH, et al. Long-Term Effect of PNPLA3 on the Aggravation of Nonalcoholic Fatty Liver Disease in a Biopsy-Proven Cohort. Clin Gastroenterol Hepatol 2023;21:1105-1107.e3. [Crossref] [PubMed]
- Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454-62. [Crossref] [PubMed]
- Petta S, Sebastiani G, Viganò M, et al. Monitoring Occurrence of Liver-Related Events and Survival by Transient Elastography in Patients With Nonalcoholic Fatty Liver Disease and Compensated Advanced Chronic Liver Disease. Clin Gastroenterol Hepatol 2021;19:806-815.e5. [Crossref] [PubMed]
- Nguyen VH, Le I, Ha A, et al. Differences in liver and mortality outcomes of non-alcoholic fatty liver disease by race and ethnicity: A longitudinal real-world study. Clin Mol Hepatol 2023;29:1002-12. [Crossref] [PubMed]


