
Glutamatergic neurons in the paraventricular nucleus of the hypothalamus participate in the regulation of visceral pain induced by pancreatic cancer in mice
Highlight box
Key findings
• Both specific destruction of glutamatergic neurons and inhibition of glutamatergic neurons in the paraventricular nucleus of the hypothalamus (PVN) alleviated visceral pain induced by pancreatic cancer.
What is known and what is new?
• The PVN is connected to the pancreas through multiple synaptic connections.
• Glutamatergic neurons in the PVN participate in the regulation of visceral pain induced by pancreatic cancer in mice.
What is the implication, and what should change now?
• Targeting PVN glutamatergic neurons may help to alleviate visceral pain in patients with pancreatic cancer.
Introduction
Pain is an unpleasant sensory and emotional affective experience associated with, or similar to, actual or potential tissue damage (1). Pain is a subjective experience that is adaptive and protective, but it can also adversely affect physical functioning, mental health, and social functioning. Chronic pain, pain that persists or recurs for more than 3 months, has been defined as a separate disorder. In developed countries in Europe and the United States, the incidence of chronic pain has reached 30% (2,3). Chronic visceral pain caused by pancreatic cancer is also a common type of pain in clinic. Abdominal pain is prevalent in patients with pancreatic cancer (4), and the incidence of advanced pain is as high as 90% (5,6). Pancreatic cancer is a highly aggressive malignancy with a poor prognosis and is the 11th most common cancer worldwide (7). There were about 90,000 new cases of pancreatic cancer in China in 2015, ranking 9th in the world (8). The visceral pain caused by pancreatic cancer not only causes serious economic burden to society and families, but also seriously affects people’s quality of life. However, the central mechanism of visceral pain in pancreatic cancer is still unclear, resulting in no specific and effective treatment for visceral pain in pancreatic cancer. Therefore, the neural mechanism of visceral pain in pancreatic cancer needs to be explored urgently.
The central circuit mechanism of visceral pain is still unclear. Existing studies have shown that different visceral pain information can be transmitted to the gray matter of the spinal cord, and then projected to higher central nervous nuclei, such as thalamus, nucleus of the solitary tract, parabrachial nucleus, midbrain periaqueductal gray matter, anterior cingulate cortex, limbic system, basal nucleus, amygdala, hypothalamus, etc. (9-11). Pancreatic cancer visceral pain has not received much attention, and only a few studies have shown that it was related to peripheral afferent nerve infiltration. However, the way that the central brain region receiving nociceptive information is still unclear. It has been found that the paraventricular nucleus of the hypothalamus (PVN) can innervate pancreatic β-cells in the endocrine partJeny (12), suggesting that PVN may be an important nucleus in the central nervous system that innervates the pancreas. In addition, the PVN is an important nerve nucleus in the hypothalamus, located in the central position of the hypothalamic-pituitary-adrenal axis (13). We previously found that intra-PVN administration of either the SK2 inhibitor apamin or PKA activator 8-Br-cAMP. can inhibit the activity of PVN neurons, thereby reducing visceral pain in mice (14). These reports indicate that the PVN may be an important nucleus in the central nervous system mechanism of visceral pain in pancreatic cancer.
In summary, in order to reveal the role and neurobiological mechanism of PVN in visceral pain of pancreatic cancer, this study will comprehensively explore the morphology, behavior, physiology and pathology of pancreatic cancer visceral pain, reveal the regulatory role of PVN in pancreatic cancer visceral pain, and clarify the therapeutic effect of PVN in the intervention of pancreatic cancer visceral pain. At the same time, because PVN is a heterogeneous nucleus and contains various types of neurons, such as GABAergic (GABA) neurons, tyrosine hydroxylase (TH) neurons, glutaminergic neurons (Glu) and corticotropin-releasing hormone (CRH) neurons, etc. (15), it is necessary to use neuron-specific experimental techniques to accurately identify which types of PVN neurons are involved in pancreatic cancer visceral pain. We found increased excitability of Glu in PVN, and both specific destruction of glutamatergic neurons and chemogenetics inhibition of glutamatergic neurons in the PVN alleviated visceral pain induced by pancreatic cancer. The results enrich and improve the regulation theory of pancreatic visceral pain, and provide new ideas for the discovery of effective targets for the treatment of visceral pain induced by pancreatic diseases. We present this article in accordance with the ARRIVE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-442/rc).
Methods
Animals
Six-week-old male C57B/6N mice were purchased from the Experimental Animal Center of Xuzhou Medical University. Mice were placed in an environment with constant temperature and humidity, a 12-hour light/dark cycle, and free access to water and food. This study was approved by the Animal Care and Use Committee of Shanghai Ninth People’s Hospital (ID: SH9H-2023-A223-SB) in compliance with the national guidelines for the care and use of animals. Male mice were randomly and blindly separated into each group according to random number table method in this study.
Mouse model of pancreatic cancer visceral pain
Mouse model of pancreatic cancer visceral pain was performed as described previously (16). mPAKPC-luc cells (purchased from Gempharmatech, Nanjing, China) were resuscitated and cultured, and frozen batches of cells were recorded. mPAKPC-luc cells at logarithmic growth stage (the 3rd to 4th generation after resuscitation) were collected, and the culture medium was removed and washed twice with Dulbecco’s phosphate-buffered saline (DPBS), and then inoculated (cell survival rate was measured before and after tumor implantation). C57BL/6 male mice were injected with mPAKPC-luc cells suspended in 100 µL mixed medium [Matrigel: phosphate-buffered saline (PBS) =1:1] at the head of the pancreas to build an orthotopic tumor model with a sterile insulin needle. Seven days after incubation, orthotopic tumor burdens were measured by the In Vivo Imaging System (IVIS). Mice with similar tumor sizes were selected for subsequent experiments to mitigate the impact of tumor size variability on behavioral assessments. Nociceptive testing was performed on 12, 15, and 18 days after injection of the mPAKPC-luc cells. The procedure of the control group was identical to that of the model group, except for the administration of mPAKPC-luc cells. When the mice in the experimental process meet the following welfare criteria, euthanasia will be conducted based on animal welfare standards, using excessive inhalation of 95% CO2 to induce death: (I) persistent diarrhea; (II) sluggishness (inability to eat or drink); (III) hunched back and lying on their side; (IV) reduced activity and symptoms of muscle atrophy; (V) difficulty breathing; (VI) progressive decrease in body temperature; (VII) paralysis and convulsions; (VIII) continuous bleeding; (IX) inability for animals to move normally due to large tumors or other reasons; and (X) inability for animals to move normally due to severe ascites or increased abdominal circumference.
Behavioral analysis
Visceral pain was assessed by abdominal mechanical hyperalgesia test and hunch score. In all cases, observations were performed by two independent observers blinded as to the experimental status of the mouse.
Abdominal mechanical hyperalgesia test
Behavioral analyses were performed as described previously with some modifications (17,18). Von Frey fibers were vertically stimulated to the left upper abdomen for about 2 s, each stimulation interval of 5 min, and repeated 10 times. Positive reactions are defined by the presence of the following behaviors: lifting, scratching, licking the abdomen, moving or jumping immediately. Response = positive response/12 mice; response frequency (%) = (positive response/10 trials) × 100. In all cases, observations were performed by two independent observers blinded as to the experimental status of the mouse.
Hunch score
The hunch score was utilized as a means of evaluating spontaneous visceral pain and was examined as described previously with some modifications (19). The scoring factors for hunch behavior were as follows—0: lack of round-back posture, showing exploratory behavior, and normal coat luster; 1: mild round-back posture, characterized by exploratory behavior and normal coat luster; 2: severe round-back posture, marked by a slight reduction in exploratory behavior, slight piloerection and intermittent abdominal contractions; 3: severe round-back posture, marked by significantly reduced exploratory behavior, moderate piloerection, and intermittent abdominal contractions; 4: severe round-back posture, characterized by little or no exploratory behavior, a full-body piloerection, and head immobility. Mice were placed individually in the center of an open field arena and observed over a 300 second period, and the hunch score was calculated by taking an average.
Pancreatic pseudorabies virus (PRV) injection
After the mice were anesthetized with pentobarbital sodium (50 mg/kg, i.p), a midline abdominal incision was made to expose the pancreas, followed by injection of PRV vector containing expressing green fluorescent protein (EGFP) (5 µL, BrainVTA, Wuhan, China) at the head of the pancreas. Mice that had been injected with the virus were put back into the cage and given plenty of water and food. After the completion of PRV injection, the state of the mice was observed every day; 5–7 days after PRV injection, the whole brain was taken out by perfusion and fixed overnight with 4% (w/v) paraformaldehyde liquid. On the second day, the brain was soaked with 30% (w/v) sucrose. Then, a series of 5–10 µm sections were cut and every fifth section analyzed by immunofluorescence and confocal microscopy.
Immunofluorescence
After implanting tumors in mice for 12 days, we stimulated the abdominal pancreas of the mice with a 0.16 g filament for approximately 2 s, with a 5-min interval between each stimulation, repeated 10 times. Half an hour later, mice were deeply anesthetized with pentobarbital sodium (50 mg/kg, i.p), followed by cardiac infusion of 20 mL normal saline and 20 mL 4% (w/v) paraformaldehyde. The brain was then taken out and fixed overnight in 4% paraformaldehyde at 4 °C. After brain preservation with 30% (w/v) sucrose, the coronal section was cut on the frozen microtome for immunofluorescence. Sections were washed 5 times, infiltrated with 0.3% Triton-X for 1 hour, and then sealed in PBS containing 5% normal goat serum and 2% bovine serum albumin for 2 hours. The brain sections were then incubated with Anti-c-Fos antibody (mouse, 1:200, Abcam, Cambridge, UK, ab208942), Anti-Glutamate antibody (Rabbit, 1:200, Sigma-Aldrich, Saint Louis, USA, G6642), Anti-GABA antibody (Rabbit, 1:200, Sigma-Aldrich, A2052), Anti-Tyrosine Hydroxylase Antibody (Rabbit, 1:200, Sigma-Aldrich, AB152), Anti-Corticotropin Releasing Factor Antibody (Rabbit, 1:200, Thermo Fisher, Waltham, USA, PA5-102356) at 4 °C for 24 h. Alexa Fluor Plus 488 Donkey anti-Mouse (1:500, Thermo Fisher, A32766) and Alexa Fluor Plus 594 Donkey anti-Rabbit (1:500, Thermo Fisher, A32754) were incubated at room temperature for 2 h. 4′,6-diamidino-2-phenylindole (DAPI, KGI Biological, Nanjing, China) was used for nuclear staining. Fluorescence signals were displayed and recorded by Zeiss laser confocal microscope (LSM80, Zeiss, Germany). For quantification of cells, three sections around the PVN were counted manually using NIH ImageJ software. The sections used were at the same coordinates for each group.
Electrophysiological experiment
After implanting tumors in mice for 12 days, we stimulated the abdominal pancreas of the mice with a 0.16 g filament for approximately 2 s, with a 5-min interval between each stimulation, repeated 10 times. Half an hour later, mice were deeply anesthetized and transcardially perfused with 95% O2 and 5% CO2 oxygenated ice-cold N-methyl-D-glucamine (NMDG) cutting solution comprising (in mM): 93 NMDG, 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 25 D-glucose, 20 hydroxyethyl piperazine ethanesulfonic acid (HEPES), 5 Na-ascorbate, 2 thiourea, 3 Na-pyruvate, 10 MgSO4, and 0.5 CaCl2, pH 7.35 with NMDG or HCl. The brain was quickly removed and immersed in the ice-cold NMDG cutting solution. PVN containing cortical slices (300 µm) were cut with a Leica VT1200s vibratome (Wetzlar, Germany), recovered at 34 °C for 10-13 min in NMDG cutting solution, and then maintained at 25 °C in oxygenated artificial cerebrospinal fluid (ACSF; in mM: 124 NaCl, 3 KCl, 2 CaCl2 1.3 MgCl2, 25 NaHCO3, 1.25 NaH2PO4, and 10 glucose) for 1 h until electrophysiological recordings.
Slices were transferred to the recording chamber and superfused with oxygenated ACSF (~3 mL/min). Slices were visualized with infrared optics using an upright microscope equipped with a 60× water-immersion lens (BX51WI, Olympus, Tokyo, Japan). We can identify glutamate neurons based on the morphology of pyramidal neurons. Pyramidal cells were distinguished from non-pyramidal cells on the basis of pyramidal-like somata and preserved apical and basal (in some neurons) dendrites. Recording pipettes were filled with a solution containing (in mM): 135 K-gluconate, 5 KCl, 0.5 CaCl2, 10 HEPES, 2 Mg-ATP, 0.1 GTP, and 5 EGTA, 300 mOsm [pH adjusted to 7.3 with potassium hydroxide (KOH)]. Membrane potentials were not corrected for the liquid-junction potential. The frequency of action potential (AP) was defined as the numbers/time window. Current-evoked APs were elicited with 400-ms current injections at ten intensities (20, 40, 60, 80, 100, 120, 140, 160, 180 and 200 pA), with a 30-s trial interval. The frequency of AP was defined as the numbers/time window. Resting membrane potentials (RMP) were measured within 1 min of break-in to the whole-cell configuration. The membrane potential at which phase plot slope exceeded 15 mV/ms was denoted threshold.
All signals were acquired with a MultiClamp 700B amplifier (Molecular Devices, Sunnyvale, USA), filtered at 2 kHz, and sampled at 10 kHz with a Digidata 1440A interface using Clampex 10.2 (Molecular Devices). Data were accepted when series resistance fluctuated within 15% of initial values (15–25 MΩ). The number of neurons and mice were indicated in the respective figure legends.
Brain stereotactic injection
Mice were anesthetized (isoflurane, induction concentration 5%, maintenance concentration 2%) and quickly fixed to a stereoscope. After the head hair shaved, the skin was cleaned with 70% alcohol, and an incision was made in the scalp, and then the periosteum tissue on the skull was wiped away with hydrogen peroxide. To inject the virus, a small hole was drilled into the skull at the target location. Virus of 100 nL was injected with a microinjector. After the injection, the microinjector was left in place for 10 min, then slowly pulled out. After the scalp was sutured, the mice were put back into the cage and given plenty of water and food. PVN: anteroposterior 0.94 mm, mediolateral ±0.20 mm, dorsoventral 5.10 mm.
Fiber photometry
Real-time Ca2+ transients were assessed via fiber photometry (ThinkerTech, Nanjing, China) to determine changes in neuronal activity. After 7 days of tumor implantation, mice with similar tumor sizes were selected for subsequent experiments using in vivo fluorescence imaging. After 9 days of tumor implantation, we microinjected 100 nL of AAV-CaMKIIα-GCaMP6m or AAV-CaMKIIα-EYFP into the PVN. An optical fiber (230 µm outside diameter, 0.37 numerical aperture, Inper, Hangzhou, China) was implanted over the region injected with GCaMp6s virus and fixed in place with dental cement. two weeks after viral expression, the calcium activity of the target neurons was monitored. Fiber photometry recordings were conducted on conscious mice that were simultaneously subjected to 0.16 g Von Frey fiber stimulation applied to the left upper abdomen. Fluorescence signals were obtained by reflecting a laser beam from a laser tube (473 nm) onto a dichroic mirror, focusing it with a 103 lens, and then coupling it to an optical commutator. Light was guided from the implanted fiber to the commutator by a 2-m optical fiber. The calcium signals were acquired with data-acquisition software (ThinkerTech) and the onset of stimulation was recorded manually. Raw signals were analyzed and processed with a Matlab program developed by Thinkertech. For each trial, the fluorescence variation was calculated as ΔF/F, where ΔF represents the value obtained by subtracting the mean of the baseline signal from the test signal and F represents standard deviation of the basal signal.
Ablation of glutamatergic neurons
After 7 days of tumor implantation, mice with similar tumor sizes were selected for subsequent experiments using in vivo fluorescence imaging. For selective ablation of PVN glutamatergic neurons, rAAV-CaMKIIα-taCasp3-T2A-TEVp-WPREs-pA or rAAV-CaMKIIα-EYFP-WPREs-pA (100 nL, BrainVTA) was injected bilaterally into the PVN of anaesthetized model mice after 9 days of tumor implantation. Behavioral assessments were conducted 14, 17, and 20 days after virus injection. After the behavioral test, the distribution of viral fluorescent protein expression in PVN was detected by fluorescence microscopy.
Chemogenetics inhibition of glutamatergic neurons
After 7 days of tumor implantation, mice with similar tumor sizes were selected for subsequent experiments using in vivo fluorescence imaging. For selective inhibition of PVN glutamatergic neurons, the PVN of mice was injected with AAV-CaMKIIα-hM4Di-mCherry or AAV-CaMKIIα-mCherry after 9 days of tumor implantation. Behavioral assessments were conducted 14, 17, and 20 days after virus injection; 40 min prior to behavioral assessments, both groups of mice received an intraperitoneal injection of 0.33 mg/mL, clozapine N-oxide (CNO) (0.2 mL/20 g). After the behavioral test, the distribution of viral fluorescent protein expression in PVN was detected by fluorescence microscopy.
Cre-dependent retrograde trans-monosynaptic rabies virus tracing strategy
Rabies virus-mediated retrograde tracing was employed to investigate the upstream regions of PVN. A mixture (150 nL, BrainVTA) of rAAV-CaMKIIa-CRE-WPRE-hGH polyA, rAAV-EF1α-DIO-H2B-EGFP-T2A-TVA-WPRE-hGHpA, and rAAV-EF1α-DIO-oRVG-WPRE-hGH pA in a 1:1:1 ratio was injected into the PVN areas of mice. After two weeks, RV-ENVA-ΔG-DsRed was injected at the same location within the PVN. Mice were sacrificed seven days following rabies virus infection. Retrograde spreading of rabies to presynaptic neurons only occurred in cells expressing both rabies virus glycoprotein (RVG) and avian sarcoma/leukosis virus subgroup A envelope protein (EnvA) cognate tumor virus receptor A (TVA). Starter cells were defined as neurons expressing both enhanced green fluorescent protein (EGFP) (from helper virus) and discosoma sp. red fluorescent protein (from rabies virus), while input cells were defined as presynaptic partners that expressed only DsRed.
Statistical analysis
All data were presented as mean ± standard deviation (SD). All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). Comparisons between multiple groups were performed by one-way analysis of variance (ANOVA) or two-way ANOVA followed by post hoc Bonferroni. Two-sample t-test was used to compare the response frequency. Wilcoxon rank sum test was used to compare hunch score. P value less than 0.05 was considered statistically significant.
Results
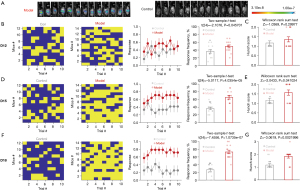
Establishment of pancreatic cancer visceral pain mouse model
In vivo fluorescence imaging of the tumor showed that the tumor cells were successfully inoculated in mice (Figure 1A). Visceral pain was assessed by abdominal mechanical hyperalgesia test and hunch score on 12, 15, and 18 days after injection of the mPAKPC-luc cells. There was a significant difference in abdominal mechanical hyperalgesia on 12 days after pancreatic injection of mPAKPC-luc cells in mice (Figure 1B), but not in hunch score (Figure 1C). There were significant differences in abdominal mechanical hyperalgesia (Figure 1D) and hunch score (Figure 1E) on 15 days after pancreatic injection of mPAKPC-luc cells in mice. There were significant differences in abdominal mechanical hyperalgesia (Figure 1F) and hunch score (Figure 1G) on 18 days after pancreatic injection of mPAKPC-luc cells, and the mean difference was further widened. The behavioral results showed that the mouse model of pancreatic cancer visceral pain was successfully established.
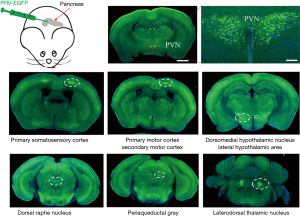
PVN innervated the pancreas through multi-stage projections
PRV, a retrograde neurotropic virus, was injected into the pancreas of mice. The PRV labeling results showed that a large number of EGFP-labeled neurons were located in the PVN (Figure 2), suggesting that the PVN in the brain innervates the pancreas through multi-stage projections. Besides, we also observed that the brain regions primarily labeled encompassed primary somatosensory cortex, primary motor cortex, secondary motor cortex, dorsomedial hypothalamic nucleus, lateral hypothalamic area, dorsal raphe nucleus, periaqueductal gray, laterodorsal thalamic nucleus.
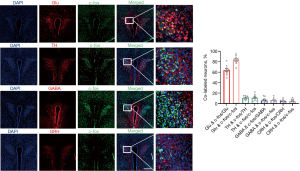
c-fos was mainly co-labeled with glutamatergic neurons
Immunofluorescence results showed that c-fos was mainly co-labeled with glutamatergic neurons, but less co-labeled with GABA neurons, CRH neurons, and TH neurons (Figure 3). Additionally, more than 90% of the PVH consists of glutamatergic neurons, while GABA neurons are less represented (20-22). Therefore, we hypothesize that visceral pain may exhibit a strong association with glutamatergic neurons. Subsequent experiments were specifically designed to investigate the role of glutamatergic neurons in this context.
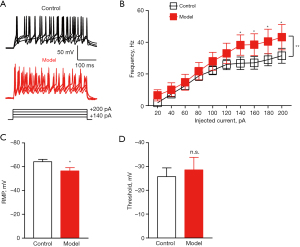
Pancreatic cancer-induced pain caused hyperactivity of PVN glutamatergic neurons
We examined the electrophysiological properties of glutamatergic neurons in the PVN using in vitro brain slices. Representative samples of APs in PVN from a control mouse and a model mouse (Figure 4A). The AP frequency was increased in orthotropic pancreatic cancer mouse model (Figure 4B). The RMP was increased in orthotropic pancreatic cancer mouse model (Figure 4C). There was no significant difference in threshold between the two groups (Figure 4D). These results suggested that pancreatic cancer-induced pain caused hyperactivity of PVN glutamatergic neurons.
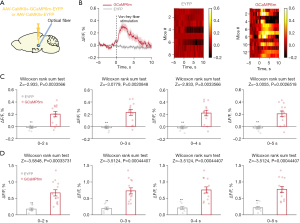
Pancreatic cancer-induced pain enhanced calcium activity in PVN glutamatergic neurons
Calcium imaging technology was used to detect the calcium activity of glutamatergic neurons. The results showed that pancreatic pain significantly enhanced calcium activity in PVN glutamatergic neurons (Figure 5A,5B). Besides, mean calcium activity and peak calcium activity both were enhanced after stimulation (0–2, 0–3, 0–4, 0–5 s) (Figure 5C,5D).
Specific destruction of PVN glutamatergic neurons alleviated visceral pain induced by pancreatic cancer
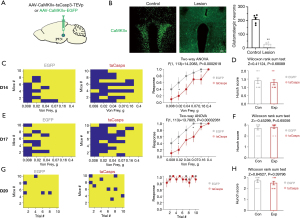
We then specifically destroyed glutamatergic neurons by taCasp3 to explore their role in visceral pain in pancreatic cancer. Consistent with the effect of ta-Casp3 on inducing cell apoptosis, taCasp3 gradually eliminated glutamatergic neurons in the PVN, resulting in an average loss of glutamatergic cells of 86.2% 20 days after virus injection (Figure 6A,6B). Visceral pain was assessed by abdominal mechanical hyperalgesia test and hunch score on 14, 17, and 20 days after injection of virus. There was a significant difference in abdominal mechanical hyperalgesia on 14 days after injection of virus in mice (Figure 6C), but not in hunch score (Figure 6D). Similarly, there were significant differences in abdominal mechanical hyperalgesia (Figure 6E), but not in hunch score (Figure 6F) on 17 days after injection of virus in mice. There were no significant differences in abdominal mechanical hyperalgesia (Figure 6G) and hunch score (Figure 6H) on 20 days after injection of virus. The behavioral results indicated that targeted ablation of PVN Glu partially mitigated the visceral pain associated with pancreatic cancer.
Chemogenetics inhibition of PVN glutamatergic neurons alleviated visceral pain induced by pancreatic cancer
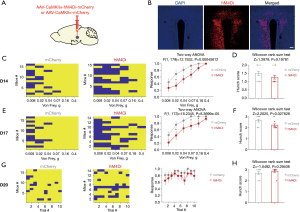
Similarly, we used chemogenetics to specifically inhibit glutamatergic neurons to explore their role in visceral pain in pancreatic cancer. Fluorescence image clarified the accuracy of the location of virus injection (Figure 7A,7B). Visceral pain was assessed by abdominal mechanical hyperalgesia test and hunch score on 14, 17, and 20 days after injection of virus. There was a significant difference in abdominal mechanical hyperalgesia on 14 days after injection of virus in mice (Figure 7C), but not in hunch score (Figure 7D). There were significant differences in abdominal mechanical hyperalgesia (Figure 7E) and hunch score (Figure 7F) on 17 days after injection of virus in mice. There were no significant differences in abdominal mechanical hyperalgesia (Figure 7G) and hunch score (Figure 7H) on 20 days after injection of virus. The behavioral results showed that specific inhibition of PVN glutamatergic neurons alleviated visceral pain induced by pancreatic cancer.
Dissection of PVN glutamatergic neurons circuit
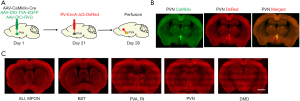
To identify the brain regions innervating PVN Glu and further explore their involvement in the regulation of visceral pain, we adopted Cre-dependent retrograde trans-monosynaptic rabies virus tracing strategy (Figure 8A). We found DsRed-labeled neurons in the lateral septal nucleus, intermediate part (SLI), medial preoptic nucleus (MPON), bed nucleus of stria terminalis (BST), paraventricular thalamic nucleus, anterior part (PVA), reticular nucleus (Rt), PVN, dorsomedial hypothalamic nucleus, dorsal part (DMD) traced from the PVN glutamatergic neurons (Figure 8B,8C).
Discussion
In this study, in order to reveal the role of PVN in pancreatic cancer visceral pain and the neurobiological mechanism, we made a pancreatic cancer visceral pain mouse model and took it as the entry point. Morphologically, retrograde tracing of PRV combined with immunohistochemistry was used to analyze the neural circuits involved in the regulation of visceral pain in the pancreatic cancer. In terms of behavior, combined with pancreatic cancer visceral pain model, the regulatory role of PVN in visceral pain was clarified. Functionally, specific type of PVN neurons were manipulated to observe the pain improvement in pancreatic cancer visceral pain model animals, in order to clarify the therapeutic effect of PVN in pancreatic cancer visceral pain. The results showed that 12 days after pancreatic injection of tumor cells, the mice developed visceral pain. The PRV injected into the pancreas was detected in the PVN. A large number of c-fos were co-labeled with glutamatergic neurons in the PVN. In vitro electrophysiological results showed that the firing frequency of glutamatergic neurons in the PVN was increased. The calcium imaging results showed that the calcium activity of glutamatergic neurons in the PVN was enhanced. Both specific destruction of glutamatergic neurons and chemogenetics inhibition of glutamatergic neurons in the PVN alleviated visceral pain induced by pancreatic cancer. Our study suggested that the PVN was involved in the of pancreatic visceral pain, and specific regulation of Glu in the PVN can alleviate pancreatic visceral pain, providing new insights for the discovery of effective targets for the treatment of pancreatic visceral pain.
The PVN integrates multiple afferents to autonomously regulate visceral sensation (23). Previous studies completed by us and others have shown that PVN can regulate visceral pain, mainly through sensitization of CRH neurons (14,24). PVN, however, was a heterogeneous nucleus that in addition to containing CRH neurons, contains many types of neurons such as GABA neurons, tyrosine hydroxylase (TH) neurons, Glu, etc. (15). The role of other types of neurons besides CRH in the regulation of visceral pain, especially pancreatic cancer visceral pain, is unknown. In order to clarify the anatomic relationship between PVN and pancreas, PRV EGFP was injected into the pancreas of mice. PRV labeling results manifested that there were a large number of EGFP-labeled neurons in the PVN. Our results suggested that the PVN can dominate the pancreas through multistage projection. Moreover, similar study have shown that PVN can dominate pancreatic endocrine β cells (12). These results demonstrated that PVN may be an important nucleus mass innervating the pancreas in the central nervous system. At the same time, our results showed that the excitability of Glu in mice with pancreatic cancer visceral pain increased, rather than CRH neurons, which were studied most in previous studies on visceral pain, through c-fos co-labeling experiment, brain patch clamp experiment, and calcium imaging experiment. In order to clarify the therapeutic effect of Glu in PVN brain region on visceral pain of pancreatic cancer, we used specific neuron activity manipulation technique, selective destruction of Glu and specific inhibition of Glu by chemogenetics to significantly alleviate visceral pain of pancreatic cancer. According to Figures 6,7, we found that the abdominal mechanical sensitivity and hunch score of mice decreased 14 and 17 days after virus injection. Therefore, we believe that inhibiting glutamatergic neurons can alleviate visceral pain. Although there was no statistically significant difference in mechanical sensitivity and hunch score between the two groups 20 days after virus injection, repeated experiments conducted ten times consistently showed a potential decreasing trend in mechanical sensitivity. However, condition of mice deteriorated 20 days after virus injection (i.e., 29 days after tumor cells injection), possibly due to health factors caused by tumors which prevented effective response to mechanical stimulation. Thus, we believe that inhibiting glutamatergic neurons can alleviate visceral pain in pancreatic cancer. These results confirm that PVN Glu are involved in the regulation of pancreatic cancer visceral pain, and the specific manipulation of Glu provides a new perspective for the treatment of pancreatic cancer visceral pain. In addition, we explored the dissection of PVN glutamatergic neurons circuit, found that some of these brain regions have been shown to be involved in visceral pain, such as BST, which further clarified the important role of PVN Glu in the regulation of visceral pain. Simultaneously, it is imperative to acknowledge that numerous brain regions have been labeled without confirmed associations with visceral pain, thereby warranting further investigation in our future research endeavors
The mechanism of visceral pain center is complex, and the key role of PVN is beyond doubt (25-27). Although our study identified the involvement of PVN Glu in the regulation of visceral pain in pancreatic cancer, it cannot be ruled out that other types of neurons are also involved, especially because the brain regions of PVN are highly heterogeneous. Therefore, in order to fully analyze the role of PVN in visceral pain, more types of neurons should be studied in different types of visceral pain animal models.
In addition, attention should be paid to whether the interaction of different types of neurons within PVN is involved in visceral pain.
Conclusions
Our study found that the PVN was involved in the pancreatic visceral pain, and specific regulation of Glu in the PVN can alleviate pancreatic visceral pain, providing new insights for the discovery of effective targets for the treatment of pancreatic visceral pain.
Acknowledgments
Funding: This study was supported by
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-442/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-442/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-442/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-442/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Animal Care and Use Committee of Shanghai Ninth People’s Hospital (ID: SH9H-2023-A223-SB) in compliance with the national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- IASP. Pain terms and definitions. (2022). Available online: https://www.iasp-pain.org/resources/terminology/#pain
- Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019;160:19-27. [Crossref] [PubMed]
- Moulin DE, Clark AJ, Speechley M, et al. Chronic pain in Canada--prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manag 2002;7:179-84. [Crossref] [PubMed]
- Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol 2016;22:9694-705. [Crossref] [PubMed]
- van Geenen RC, Keyzer-Dekker CM, van Tienhoven G, et al. Pain management of patients with unresectable peripancreatic carcinoma. World J Surg 2002;26:715-20. [Crossref] [PubMed]
- Lindsay TH, Jonas BM, Sevcik MA, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain 2005;119:233-46. [Crossref] [PubMed]
- Carvajal G. Pancreatic Cancer Related Pain: Review of Pathophysiology and Intrathecal Drug Delivery Systems for Pain Management. Pain Physician 2021;24:E583-94.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Binetti M, Lauro A, Golfieri R, et al. False in Name Only-Gastroduodenal Artery Pseudoaneurysm in a Recurrently Bleeding Patient: Case Report and Literature Review. Dig Dis Sci 2019;64:3086-91. [Crossref] [PubMed]
- Farmer AD, Coen SJ, Kano M, et al. Psychophysiological responses to visceral and somatic pain in functional chest pain identify clinically relevant pain clusters. Neurogastroenterol Motil 2014;26:139-48. [Crossref] [PubMed]
- Grundy L, Erickson A, Brierley SM. Visceral Pain. Annu Rev Physiol 2019;81:261-84. [Crossref] [PubMed]
- Rosario W, Singh I, Wautlet A, et al. The Brain-to-Pancreatic Islet Neuronal Map Reveals Differential Glucose Regulation From Distinct Hypothalamic Regions. Diabetes 2016;65:2711-23. [Crossref] [PubMed]
- Chen CR, Zhong YH, Jiang S, et al. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. Elife 2021;10:e69909. [Crossref] [PubMed]
- Ji NN, Du L, Wang Y, et al. Small-Conductance Ca(2+)-Activated K(+) Channels 2 in the Hypothalamic Paraventricular Nucleus Precipitates Visceral Hypersensitivity Induced by Neonatal Colorectal Distension in Rats. Front Pharmacol 2020;11:605618. [Crossref] [PubMed]
- Chen S, Xu H, Dong S, et al. Morpho-Electric Properties and Diversity of Oxytocin Neurons in Paraventricular Nucleus of Hypothalamus in Female and Male Mice. J Neurosci 2022;42:2885-904. [Crossref] [PubMed]
- Wang M, Wu M, Liu X, et al. Pyroptosis Remodeling Tumor Microenvironment to Enhance Pancreatic Cancer Immunotherapy Driven by Membrane Anchoring Photosensitizer. Adv Sci (Weinh) 2022;9:e2202914. [Crossref] [PubMed]
- Selvaraj D, Hirth M, Gandla J, et al. A mouse model for pain and neuroplastic changes associated with pancreatic ductal adenocarcinoma. Pain 2017;158:1609-21. [Crossref] [PubMed]
- Yu D, Zhu J, Zhu M, et al. Inhibition of Mast Cell Degranulation Relieves Visceral Hypersensitivity Induced by Pancreatic Carcinoma in Mice. J Mol Neurosci 2019;69:235-45. [Crossref] [PubMed]
- Sevcik MA, Jonas BM, Lindsay TH, et al. Endogenous opioids inhibit early-stage pancreatic pain in a mouse model of pancreatic cancer. Gastroenterology 2006;131:900-10. [Crossref] [PubMed]
- Vong L, Ye C, Yang Z, et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 2011;71:142-54. [Crossref] [PubMed]
- Xu Y, Wu Z, Sun H, et al. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab 2013;18:860-70. [Crossref] [PubMed]
- Ziegler DR, Cullinan WE, Herman JP. Organization and regulation of paraventricular nucleus glutamate signaling systems: N-methyl-D-aspartate receptors. J Comp Neurol 2005;484:43-56. [Crossref] [PubMed]
- Tsushima H, Zhang Y, Muratsubaki T, et al. Oxytocin antagonist induced visceral pain and corticotropin-releasing hormone neuronal activation in the central nucleus of the amygdala during colorectal distention in mice. Neurosci Res 2021;168:41-53. [Crossref] [PubMed]
- Huang ST, Wu K, Guo MM, et al. Glutamatergic and GABAergic anteroventral BNST projections to PVN CRH neurons regulate maternal separation-induced visceral pain. Neuropsychopharmacology 2023;48:1778-88. [Crossref] [PubMed]
- Ji NN, Kang J, Hua R, et al. Involvement of dopamine system in the regulation of the brain corticotropin-releasing hormone in paraventricular nucleus in a rat model of chronic visceral pain. Neurol Res 2018;40:650-7. [Crossref] [PubMed]
- Tang HL, Zhang G, Ji NN, et al. Toll-Like Receptor 4 in Paraventricular Nucleus Mediates Visceral Hypersensitivity Induced by Maternal Separation. Front Pharmacol 2017;8:309. [Crossref] [PubMed]
- Song Y, Meng QX, Wu K, et al. Disinhibition of PVN-projecting GABAergic neurons in AV region in BNST participates in visceral hypersensitivity in rats. Psychoneuroendocrinology 2020;117:104690. [Crossref] [PubMed]


