
Prognostic impact of tumor marker kinetics and normalization during neoadjuvant chemotherapy for pancreatic cancer
We congratulate Dr. Newhook and colleagues for their recently published study entitled “Prognosis Associated With CA19-9 Response Dynamics and Normalization During Neoadjuvant Therapy in Resected Pancreatic Adenocarcinoma” in Annals of Surgery (1).
Pancreatic ductal adenocarcinoma (PDAC) remains the most lethal type of human cancer due to its high chemoresistance and invasiveness (2). In resected PDAC, the median overall survival (OS) has increased from 22.1 to 35 months during the past 10 years, largely owing to improvements in adjuvant therapies (3,4). On the other hand, surgical resection offers the only chance of cure, but surgery can be associated with significant morbidity and decreased activities of daily living (ADL), especially in the Whipple procedure (pancreaticoduodenectomy). Recently, the importance of preoperative chemotherapy even in operable PDAC has been increasingly recognized (5). Although molecular markers are used more frequently in patients selected for systemically targeted agents, only imaging modalities are used to stage patients and assess their suitability for surgical resection. Decisions regarding initial surgery or neoadjuvant approaches are made in the absence of biological indicators to assess the risk of aggressive tumors or occult metastatic disease. In this unique study, the authors aim to establish a new classification system (A-B-C-D-E) by analyzing the dynamic changes of serum carbohydrate antigen 19-9 (CA19-9) during neoadjuvant therapy (NT) in PDAC patients and assess its relationship with post-resection survival. The research reveals that, compared to the sole normalization of CA19-9, this classification system more accurately predicts post-resection survival in PDAC patients. It provides valuable information for patients, caregivers, and clinicians regarding potential surgical outcomes. Overall, this novel and practical classification scheme offers more instructive insights for treatment decisions in PDAC patients. It was described as the “A”lways decreasing to normal; “B”idirectional to normal; “C”onsistently normal; “D”ecreasing without normalization; and “E”levating without normalization (Figure 1). Then, the prognostic outcomes of the different kinetics in response to NT were ordered as follows, revealing a longest-to-shortest type ranking of OS as A→B→C→D→E; relapse-free survival as B→A→D→C→E. Thus, Dr. Newhook and colleagues successfully elucidated the prognostic impact of tumor marker kinetics and normalization during neoadjuvant chemotherapy for PDAC.
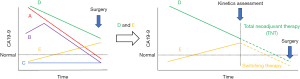
In a cohort study of 4,041 patients treated with upfront surgery and 1,175 patients treated with NT (79.4% multiagent NT, 20.6% single-agent NT), multiagent NT followed by resection is associated with improved survival compared to upfront surgery (6). Furthermore, adjuvant chemotherapy (AC) following multiagent NT and resection in patients with PDAC was associated with significant survival benefit compared with that in patients who did not receive AC. These findings suggest that patients with aggressive tumors may benefit from AC to achieve prolonged survival, even after multiagent NT and curative-intent resection (7), and may support that importance of the possibly normalization of CA19-9 with a total NT or switching chemotherapeutic agents (Figure 1).
Dr. Zhao and colleagues (8) investigated the patients with PDAC (n=806) were split into two groups with normal (≤37 U/mL) and elevated (>37 U/mL) CA19-9, and they then developed the clinicopathological features, survival, and recurrence patterns were compared between two groups. PDAC with normal CA19-9 were less likely to have lymph node metastasis, angiolymphatic invasion, intrapancreatic nerve invasion, anterior plasma membrane invasion, and invasion of peripheral tissues/organs (distal bile ducts, duodenum, or splenic artery). After propensity score matching, PDAC with normal CA19-9 levels (≤37 U/mL) was associated with significantly superior OS after resection. In addition, the CA19-9 ≤37 U/mL group had significantly lower rates of local recurrence (35.57% vs. 52.35%, P=0.004), distant recurrence (42.95% vs. 60.4%, P=0.003), and mixed recurrence (5.37% vs. 29.53%, P<0.000) compared to the CA19-9 >37 U/mL group. A large multicenter study demonstrated that improved biochemical response (≥50% reduction in CA19-9) and pathological response [pathological complete response (pCR) and pathological partial response (pPR)] to NT with FOLFIRINOX or gemcitabine/nab-paclitaxel resulted in better OS, local recurrence-free survival and metastasis-free survival biochemical response (<50% reduction in CA19-9), and limited pathological response (pLR) compared to patients. These results suggest that pCR, pPR, and CA19-9 reduction of ≥50% or normalization in response NT with FOLFIRINOX or gemcitabine/nab-paclitaxel can be alternative prognostic markers of OS (9).
CA19-9 is the most commonly used biomarker for pancreatic cancer, but its detection sensitivity and specificity are not high enough. The data derived from the analysis of serum samples from 362 patients by Dr. Dong et al. could provide valuable information for the early diagnosis of PDAC using CA19-9, periostin (POSTN) as marker groups in clinical work. At the same time, this study demonstrated that POSTN and CA242 are potential diagnostic serum biomarkers to complement CA19-9 in the detection of PDAC (10). Carcinoembryonic antigen (CEA) is also commonly used tumor marker for gastrointestinal malignancies. It was originally developed for pancreatic cancer and used throughout the 1970–1980 period before the introduction of CA19-9. Currently, CEA is the standard tumor marker for screening and predicting prognosis in colorectal cancer (11). Elevated CEA levels prior to treatment using standard diagnostic thresholds provide important prognostic information for patients with pancreatic cancer. Also, further studies to determine whether CEA has predictive value for treatment modalities and chemotherapy regimens are warranted (12). Miyata et al. (13) compared the prognostic impact of preoperative serum CA19-9, CEA, s-pancreas antigen-1 (SPan-1), duke pancreatic monoclonal antigen type 2 (DUPAN-II) levels and their positive number was scored as the preoperative tumor marker index (pre-TI) in patients with resectable PDAC. Then, high pre-TI is an independent worse prognostic factor and the numerical scoring of pre-TI would be a useful biomarker for predicting the prognostic outcome of PDAC after pancreatectomy. Then, the preoperative positive number of tumor markers could be a potent predictive marker of prognostic outcomes for patients with resections for PDAC. Kondo et al. (14) examined pre- and post-operative serum CA19-9, SPan-1, and DUPAN-II levels in patients with resectable PDAC, and then elucidated that elevated postoperative CA19-9 (≥37 IU/mL) was the strongest predictive marker of poor survival among preoperative and postoperative serum CA19-9, SPan-1, and DUPAN-II levels. Kato et al. (15) elucidated that high CEA level (>7.2 ng/mL) after NT is a worse prognostic indicator for localized PDAC. Then, they insisted that locally advanced PDAC with a high CEA level even after NT should still be recognized as a systemic disease. Although Newhook et al. (1) conducted this study using single tumor marker of CA19-9, a further kinetic approach using multi-tumor markers also may be useful to fight this deadly disease.
A new era of preoperative NT and surgery for pancreatic cancer is opening. Prospective evaluation of the diagnosis of PDAC and treatment options, including chemotherapy and surgery, is needed and used to design scientifically sound treatment strategies. The study conducted by Dr. Newhook et al. (1) not only provides valuable information for preoperative NT with prognostic relevance but also precision medicine using CA19-9 kinetics for PDAC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-662/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Newhook TE, Vreeland TJ, Griffin JF, et al. Prognosis Associated With CA19-9 Response Dynamics and Normalization During Neoadjuvant Therapy in Resected Pancreatic Adenocarcinoma. Ann Surg 2023;277:484-90. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med 2018;379:2395-406. [Crossref] [PubMed]
- Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016;388:248-57. [Crossref] [PubMed]
- Ye M, Zhang Q, Chen Y, et al. Neoadjuvant chemotherapy for primary resectable pancreatic cancer: a systematic review and meta-analysis. HPB (Oxford) 2020;22:821-32. [Crossref] [PubMed]
- Sugawara T, Rodriguez Franco S, Sherman S, et al. Neoadjuvant Chemotherapy Versus Upfront Surgery for Resectable Pancreatic Adenocarcinoma: An Updated Nationwide Study. Ann Surg 2024;279:331-9. [Crossref] [PubMed]
- Sugawara T, Rodriguez Franco S, Sherman S, et al. Association of Adjuvant Chemotherapy in Patients With Resected Pancreatic Adenocarcinoma After Multiagent Neoadjuvant Chemotherapy. JAMA Oncol 2023;9:316-23. [Crossref] [PubMed]
- Zhao Y, Wang C. Clinicopathological Features, Recurrence Patterns, and Prognosis of Pancreatic Adenocarcinoma with Normal Serum CA19-9. A Consecutive Series of 154 Cases from a Single Institute. J Gastrointest Surg 2020;24:855-65. [Crossref] [PubMed]
- Macedo FI, Ryon E, Maithel SK, et al. Survival Outcomes Associated With Clinical and Pathological Response Following Neoadjuvant FOLFIRINOX or Gemcitabine/Nab-Paclitaxel Chemotherapy in Resected Pancreatic Cancer. Ann Surg 2019;270:400-13. [Crossref] [PubMed]
- Dong D, Jia L, Zhang L, et al. Periostin and CA242 as potential diagnostic serum biomarkers complementing CA19.9 in detecting pancreatic cancer. Cancer Sci 2018;109:2841-51. [Crossref] [PubMed]
- Carriquiry LA, Piñeyro A. Should carcinoembryonic antigen be used in the management of patients with colorectal cancer? Dis Colon Rectum 1999;42:921-9. [Crossref] [PubMed]
- Lee KJ, Yi SW, Chung MJ, et al. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med J 2013;54:643-9. [Crossref] [PubMed]
- Miyata T, Hayashi H, Yamashita YI, et al. Prognostic Value of the Preoperative Tumor Marker Index in Resected Pancreatic Ductal Adenocarcinoma: A Retrospective Single-Institution Study. Ann Surg Oncol 2021;28:1572-80. [Crossref] [PubMed]
- Kondo N, Murakami Y, Uemura K, et al. Comparison of the prognostic impact of pre- and post-operative CA19-9, SPan-1, and DUPAN-II levels in patients with pancreatic carcinoma. Pancreatology 2017;17:95-102. [Crossref] [PubMed]
- Kato H, Kishiwada M, Hayasaki A, et al. Role of Serum Carcinoma Embryonic Antigen (CEA) Level in Localized Pancreatic Adenocarcinoma: CEA Level Before Operation is a Significant Prognostic Indicator in Patients With Locally Advanced Pancreatic Cancer Treated With Neoadjuvant Therapy Followed by Surgical Resection: A Retrospective Analysis. Ann Surg 2022;275:e698-707.


