
Evaluation of the economic impact of the robotic approach in major and postero-superior segment liver resections: a multicenter retrospective analysis
Highlight box
Key findings
• Robotic liver surgery (RLS) is associated with higher intraoperative costs, primarily related to surgical equipment expenses. However, these were slightly offset by the postoperative savings resulting from significantly reduced length of stay.
What is known and what is new?
• RLS has gained prominence in minimally invasive hepatobiliary surgery, accounting for less than 8% of in Europe, with financial considerations being a limiting factor. Prior studies have reported conflicting results on the cost-effectiveness of RLS primarily due to variations in cost calculation methods.
• This study contributes comprehensive economic analysis, based on real-world evidence from Italian centers, utilizes a time-driven activity-based costing model, revealing RLS’s potential cost-effectiveness despite higher intraoperative costs.
What is the implication, and what should change now?
• Insights into perioperative outcomes, and costs associated with open, laparoscopic, and robotic liver surgeries aid informed decision-making in clinical practice. Hospitals and healthcare systems should evaluate the cost implications of adopting RLS, considering both intraoperative and postoperative expenses. Anticipated reductions in acquisition costs and increased proficiency in RLS are expected to contribute to its favorability. Continuous efforts in training and protocol development are needed for a balanced assessment of clinical benefits, economic implications, and ongoing advancements in the field.
Introduction
In the expanding era of minimally invasive surgery, the robot-assisted technique is emerging as an important contribution in the field of hepatobiliary surgery (1). The role of robotic liver surgery (RLS) as a valid alternative to laparoscopy is documented and there is a growing body of evidence regarding its feasibility and safety (2). However, the precise role of the robotic approach in the whole scenario of liver surgery is not fully determined (3). Similarly to laparoscopy, RLS provides superior peri-operative outcomes in comparison to the open approach, including lower blood loss, decreased morbidity, shortened length of hospitalization and earlier return to daily activities (4). Thereby, RLS has shown adequacy for the treatment of malignant diseases, even providing advantages to achieve the oncological radicality while preserving the benefits of minimally invasiveness (5).
Since its introduction, robotic technology has been designed and has progressively evolved to face laparoscopic setbacks (6). While a major drawback of laparoscopic surgery is reduced dexterity with poor extent of motions and difficulty performing complex tasks such as suturing or vascular resections (7), the robotic platform has provided instruments with high degrees of freedom, a stable three-dimensional (3D) vision, algorithms suppressing the hand tremor, and enhanced ergonomics for the console surgeon (8-10). It is suggested that the robotic approach may facilitate more difficult liver resections due to freely articulating and angling instruments, such as resections of the postero-superior segments (namely, segments 1, 7, 8, and 4a), major liver hepatectomies (defined as three or more Couinaud segments) and those requiring extensive hilar dissection or biliary reconstructions, thus reducing the risk of conversion and conversion related risks of morbidity and mortality (11-14).
Despite these technological advantages, the robotic technique accounts for less than 8% of all minimally invasive hepatectomies. Among factors limiting its spread, the most mentioned in literature is cost. In a recent survey conducted across 103 European liver centres to assess the adoption of the robotic approach, respondents identified uncertain effect on financial expenditure as the predominant obstacle, with 80% citing it as a significant disadvantage (15).
Previous analysis on costs of RLS has yielded controversial results about the balance between costs and benefits deriving from its implementation. On the one side, the techniques used to calculate costs vary greatly, potentially impacting the final outcome (16-21). Additionally, the existing literature on this topic mostly compare broad categories of liver resections without considering the initial purchase costs of the robotic platform, which is associated with a significant increase in hospital resource utilization. As such the actual knowledge is not sufficient to draw firm conclusions on this topic.
Considering these premises, the aim of the study is to perform a rigorous economic analysis, through a time-driven activity-based costing (TD-ABC) model, in order to compare open, laparoscopic and robotic approaches using real world data coming from two high volume Italian centres with well-established programs of liver resections. Provided the overall clinical benefit compared with laparoscopy already described, the analysis is restricted to procedures with high degree of technical complexity. In particular, in a prior investigation, Cipriani et al. conducted a comparative analysis of laparoscopic and robotic liver surgeries, stratifying the resections based on varying levels of difficulty. The findings support the theoretical advantages of robotics particularly in facilitating postero-superior and major liver resections (2). The result of this study prompted the authors to specifically concentrate the current analysis on this specific setting to address the issue of comparative costs of open vs. laparoscopy vs. robotic approach as a primary endpoint.
Secondary endpoint is the comparative evaluation of short-term clinical outcomes and the analysis of real-life allocation of cases to each available approach (i.e., open, laparoscopic, robotic).
Methods
Study design
For the present investigation, the study population was identified from prospectively maintained databases at two Italian high-volume hepatobiliary centres. Consecutive liver resections performed for primary or secondary liver tumours and benign conditions were identified by retrospectively reviewing the database of surgical activity performed at San Raffaele Hospital (Milan, Italy) and at Gemelli Hospital (Rome, Italy). Major hepatectomies and postero-superior liver resections performed from February 2021 to April 2022 with open, laparoscopic, or robotic approaches constituted the study population. According to the Brisbane 2000 Nomenclature, major liver resections were defined as the resection of three or more liver segments (22). Postero-superior segments were defined following the Couinaud segmental anatomy of the liver (segments 1, 7, 8, and 4a) (23). The analysis was restricted to a 1-year time frame to reduce the influence of major variations in the inflation power over time on final results and to consider a homogeneous pool of procedures carried out with a standardized technique and perioperative management.
Exclusion criteria were the following:
- Age under 18;
- Non elective admission;
- Two stage hepatectomies, including Associating Liver Partitioning and Portal vein ligation for Staged hepatectomy (ALPPS);
- Cyst unroofing and pericystectomies.
The type of procedure scheduled (i.e., major hepatectomies and postero-superior liver resections) was decided without adjustments based on the surgical approach (open/laparoscopic/robotic). The following disease characteristics were considered as exclusion criteria for a minimally invasive (laparoscopic or robotic) approach over the entire period:
- Lesions strictly adjacent or infiltrating the hepatocaval confluence or inferior vena cava;
- Lesions with presumed infiltration of the hepatic vein of the future liver remnant;
- Patients with portal vein thrombosis requiring portal vein thrombectomy;
- Patients with more than 10 liver lesions and/or requiring more than 10 resection areas;
- Anaesthesiologic contraindications to pneumoperitoneum (e.g., severe cardio-pulmonary disease).
The bi-institutional cohort of liver resections was stratified into three study groups according to the approach defined by the surgical team: open, laparoscopic, and robotic. With regard to the occurrence of conversion to an open procedure during laparoscopic or robotic resections, the primary analysis relied on the intention-to-treat analysis (ITT-A; i.e., retaining the converted cases in the laparoscopic and in the robotic group) to reflect at best the real clinical setting, where conversion is required in a variable proportion of patients. The reasons for conversion have been classified in: technical difficulties, oncological radicality, intraoperative hemorrhage, anesthesiologic issues, adhesions due to previous surgery and injury to adjacent organs.
The study population derived from the Italian “I GoMILS” registry. Ethical approval for the registry was granted by the Ethics Committee of the promoting center and shared among the participants (IGOMILS-OSR of March 6, 2014—available at: https://www.cr-technology.com/igomils/eclinical/website/documents.aspx). Written consent from subjects was waived.
This study adheres to the principles outlined in the Declaration of Helsinki (as revised in 2013).
Preoperative and intraoperative management
Prior to surgery, a standard staging was performed and this included routine blood tests, computed tomography of the abdomen with triphasic liver contrast enhancement, and/or liver specific contrast magnetic resonance imaging. All cases underwent a comprehensive evaluation in formal weekly institutional multidisciplinary meetings, involving the surgical team, pathologist, radiologist, oncologist, anaesthetists, and navigator nurse. These meetings facilitated the development of an overall operative plan, considering patient characteristics, comorbidities, and the pathological, anatomical, and radiological features of the disease, guiding the selection of the surgical approach. Strict adherence to recent exclusion criteria for minimally-invasive liver surgery was maintained. The criteria and process for selecting patients for each surgical approach were not affected by patients’ treatment preferences (24).
Robotic approach: The Da Vinci® Xi robotic platform (Intuitive Surgical, USA) was utilized in all robotic cases. The patient received general anaesthesia and the first surgeon operated at the console while the assistant was standing between the patient’s legs. A laparoscopic 10 mm trocar was positioned in an infraumbilical right position, and pneumoperitoneum was induced. Four robotic trocars were placed following a standardized configuration based on the planned resection. The standard robotic instrumentation for liver resections included: prograsp forceps, Maryland bipolar forceps, and monopolar scissors. Robo-Lap approach was used in selected cases to address the task of parenchymal transection (25). Specifically, 55.3% of patients who underwent RLS were performed with Robo-Lap approach. Both the referring centers object of the analysis used the Misonix® device.
Laparoscopic approach: with the patient under general anaesthesia, the surgical team followed the French position with the first surgeon positioned between the patient’s legs and the first and the second assistant on the left and on the right side of the patient, respectively. As described in previous reports, five ports were placed in an inverted J-shaped standard configuration (26,27). Parenchymal transection was performed by combining ultrasonic dissector and bipolar forceps as described in details in previous works (28).
Open approach: with the patient under general anaesthesia, a xipho-supraumbilical incision extending to the right subcostal area was made in subjects undergoing laparotomy. During the procedure, intraoperative ultrasound was routinely performed to assess the anatomy of the liver, confirm resectability and evaluate the relationship between the lesion and the main vascular and biliary structures within the liver parenchyma.
Lastly, regardless of the approach employed, primary extraparenchymal vascular control was always achieved before transection (Pringle maneuver).
Perioperative endpoints and definitions
Open, laparoscopic, and robotic liver resections were compared for the assessment of feasibility and efficacy, following parameters of short-term outcomes.
Intraoperative outcomes included: operative time (minutes), blood loss (mL), red blood cells and fresh frozen plasma (FFP) transfusions and amount (mL), surgical radicality rate (R0), conversion rate (for laparoscopic and open groups). Operative time was calculated as the time between laparotomy and skin suture for the open group and the time between pneumoperitoneum induction and port-site closure for minimally invasive groups (laparoscopic and robotic groups). Intraoperative blood loss was measured by subtraction. Complications were classified according to the Clavien-Dindo classification (29).
Postoperative outcomes included in-hospital mortality and morbidity rate; type and severity of complications (general and liver-specific), reoperation rate, blood products (red cells and FFP), transfusions and amount (mL), postoperative investigations (radiological/endoscopic), administration of medical agents, postoperative radiological/endoscopic procedures (including radio-guided drainage of collections, ascites or pleural effusion; endoscopic retrograde cholangiopancreatography ± insertion of biliary stent), length of stay (days). Specific definitions of liver complications were adopted: liver failure as an increased international normalized ratio and concomitant hyperbilirubinemia on or after postoperative day (POD) 5 (30); ascites as an abdominal drainage above 10 mL/kg body weight/day after POD 3 (31); bile leak as a bilirubin concentration in the drainage above three-fold of serum total bilirubin on or after POD 3, or the need for radiologic or operative intervention from a biliary collection or bile peritonitis (32). Resection margins were defined into R0 and R1 (33).
Economic analysis
The economic analysis took into consideration contributors of hospital costs as follows:
- Intraoperative expenses: including operating room charges per minute, equips (surgeons, anaesthetists, nurses) per minute, disposable devices per piece, sterilization/maintenance of reusable devices, anaesthetic medical agents per posological unit, blood transfusion requirements (mL), histopathological exam (frozen sections and final) per service.
- Postoperative expenses: including blood transfusion requirement (mL), antibiotic therapy per posological unit, postoperative imaging per service, any reintervention (radiological and/or endoscopic and/or surgical) per service, and ward/intensive care unit (ICU) bed per night.
Investigations and clinical evaluations in the setting of the preoperative assessments and indirect costs were excluded from the analysis.
Intraoperative and postoperative expenses were calculated using the TD-ABC methodology described by Kaplan and Anderson at the Harvard Business School, considered the gold standard for cost determination studies (34). Briefly, TD-ABC examines actual operational processes across the entire care pathway and has been shown to be more accurate in cost analyses compared to traditional accounting methods. TD-ABC provides patient specific data on the cost of personnel and supply resources consumed by each patient. Furthermore, provided the temporal analysis of TD-ABC, it allows to estimate the unit cost of supplying capacity (e.g., cost per minute of a surgeon’s time) and utilizes time equations to estimate the time spent performing each activity (35). This technique is based on the concept that the performance of a service consumes activities which then consume resources. TD-ABC attempts to assign costs to each of these activities and/or resources so that total costs can be better understood and managed. It differs from traditional accounting in that it is based on the activities that drive costs. This allows one to manage processes by having clearer understanding of what drives costs and how increases in efficiency affect costs. Many quality improvement techniques also break process into discreate units. This is done to standardize processes, improve then, and eliminate unnecessary variability (36). Process maps were used to determine TD-ABC for all cases included in the study. Indirect costs were excluded from the analysis.
Personnel costs were computed using salary data obtained from the national collective labor agreement. By dividing the calculated cost by the capacity in minutes, a per-minute capacity cost rate was provided for each professional involved in the operating room. The capacity cost rate was multiplied by the mean operative time in order to define the total cost.
Costs associated with disposable and reusable devices were determined using the regional calls for tenders; postoperative investigations costs were obtained from the Italian national outpatient tariffs; the antibiotic therapy costs were extrapolated from the Italian medicine agency transparency lists using an average posology from the summary of product characteristics (SPCs).
Costs of sterilization of reusable devices, anaesthetic medical agents, histopathological exam, and ward/ICU bed per night were identified by an extensive review of the scientific literature (37-40).
Regarding the blood products transfusion costs, calculations were determined according to expert opinions from the recruiting centers. A specific TD-ABC model, developed from the input received of physicians operating in transfusion centers, has been established in order to assign value to staff time, disposables, hemovigilance, tests, and transportation expenses.
The cost of conversion for the two minimally invasive groups was express as an additional cost, calculated as a percentage. This was determined by taking the ratio between the mean cost of converted resections to the mean cost of completed resections.
A comparative sub-analysis of intra-, postoperative and total costs was performed for patients who had no postoperative complications.
Data costs inputs were extracted and audited by an expert in health economics from the Postgraduate School of Health Economics and Management (ALTEMS) of Università Cattolica del Sacro Cuore, Rome.
All costs calculations were performed in Euro and results are expressed in Euro.
Statistics
Demographic data, surgical procedures and the postoperative course were analysed. Before the comparison, the homogeneity of the sample was tested for the baseline characteristics. Variables used were: age (years); gender (male/female); ASA score (1–4); diagnosis (benign/malignant); size of the largest lesion (mm); background liver (healthy/steatosis/fibrosis-cirrhosis); liver function according to the Child-Pugh grade (A/B); previous abdominal surgery (yes/no); extent of the liver resection (minor/major); type of hepatectomy (major hepatectomy/postero-superior sectionectomy); associated hilar lymphadenectomy (LND) (yes/no); associated biliary reconstruction (yes/no). Only those patients with completed perioperative data available at the end of recruitment were selected for comparison.
For continuous variables, median values with interquartile ranges were considered since their distribution was skewed (Shapiro-Wilk test); categorical variables were expressed as absolute values and proportions. Continuous variables were compared using the non-parametric Kruskal-Wallis test; categorial variables were compared trough the chi-square test. Continuous variables related costs are expressed as mean with standard deviation, absolute difference, percentage difference, and are compared using the analysis of variance (ANOVA). Statistical significance was set at P<0.05 and hypothesis tests were two-sided. Statistical analysis was performed using Jamovi software (version 2.3.21 for Windows).
Results
Patient characteristics
Two hundred and seventy-two patients within the study period were included in the analysis. There were 124 (46%) patients who had open liver surgery (OLS), 101 (37%) patients who had laparoscopic liver surgery (LLS), and 47 (17%) patients who underwent RLS.
Comorbidity rate was similar among all three cohorts, as demonstrated by the same patient’s stratification based on the American Society of Anaesthesiologists score.
Indications for surgery were dissimilar: a higher proportion of benign disease was present in the RLS group whereas malignant disease were more frequent in the other two groups (76.60% for RLS vs. 92.08% for LLS vs. 95.97% for OLS, P<0.001). Liver lesions robotically approached have shown larger median size than the other two groups (40 vs. 30 mm for LLS vs. 30 mm for OLS, P<0.001). Even though a statistically different distribution of the background liver was observed, Child-Pugh grade did not differ among the three groups. The rate of previous abdominal surgery was significantly higher in the RLS group (78.72% vs. 56.44% for LLS vs. 50% for OLS, P=0.003).
In terms of the type of hepatectomy the OLS and the RLS groups had more major hepatectomies than the LLS group, and inversely the LLS group had more postero-superior liver resections than the other two groups (P=0.018). However, the proportion of hilar lymphadenectomies and biliary reconstructions did not differ between groups. Complete baseline characteristics are shown in Table 1.
Table 1
| Patient baseline characteristics | OLS (n=124) | LLS (n=101) | RLS (n=47) | P value |
|---|---|---|---|---|
| Diagnosis | ||||
| Benign | 5 (4.03) | 8 (7.92) | 11 (23.40) | <0.001 |
| Adenoma | 1 (0.81) | 0 | 3 (6.38) | 0.008 |
| Haemangioma | 3 (2.42) | 4 (3.96) | 2 (4.26) | 0.751 |
| Other | 2 (1.61) | 4 (3.96) | 6 (12.77) | 0.115 |
| Malignant | 119 (95.97) | 93 (92.08) | 36 (76.60) | <0.001 |
| HCC | 9 (7.26) | 18 (17.82) | 10 (21.28) | 0.017 |
| iCCA | 17 (13.71) | 8 (7.92) | 9 (19.15) | 0.135 |
| pCCA | 14 (11.29) | 6 (5.94) | 2 (4.26) | 0.195 |
| CRLM | 73 (58.87) | 57 (56.44) | 9 (19.15) | <0.001 |
| Other | 6 (4.84) | 4 (3.96) | 6 (12.77) | 0.085 |
| Age (years) | 66 (56–71) | 67 (57–73) | 66 (59–75) | 0.650 |
| Gender | ||||
| Female | 47 (37.90) | 41 (40.59) | 22 (46.81) | 0.570 |
| Male | 77 (62.10) | 60 (59.41) | 25 (53.19) | |
| ASA score | 0.331 | |||
| ASA 1 | 10 (8.06) | 7 (6.93) | 5 (10.64) | |
| ASA 2 | 60 (48.39) | 59 (58.42) | 25 (53.19) | |
| ASA 3 | 48 (38.71) | 35 (34.65) | 15 (31.91) | |
| ASA 4 | 6 (4.84) | 0 | 2 (4.26) | |
| Size of the biggest lesion (mm) | 30 (17–56) | 30 (20–45) | 40 (25–66) | <0.001 |
| Background liver | 0.002 | |||
| Healthy | 109 (87.90) | 81 (80.20) | 30 (63.83) | |
| Steatosis | 4 (3.23) | 9 (8.91) | 10 (21.28) | |
| Fibrosis/cirrhosis | 11 (8.87) | 11 (10.89) | 7 (14.89) | |
| Child-Pugh grade | 0.090 | |||
| Child-Pugh A | 124 (100.00) | 101 (100.00) | 46 (97.87) | |
| Child-Pugh B | 0 | 0 | 1 (2.13) | |
| Previous abdominal surgery | 62 (50.00) | 57 (56.44) | 37 (78.72) | 0.003 |
| Type of the hepatectomy | 0.018 | |||
| Major hepatectomy | 56 (45.16) | 28 (27.72) | 21(44.68) | |
| Postero-superior sectionectomy | 68 (54.84) | 73 (72.28) | 26 (55.32) | |
| Associated hilar lymphadenectomy | 22 (17.74) | 12 (11.88) | 11 (23.40) | 0.190 |
| Associated biliary reconstruction | 12 (9.68) | 6 (5.94) | 2 (4.26) | 0.380 |
The distribution of dichotomous categorical variables is expressed by n (%). Continuous variables are expressed as median (interquartile range). OLS, open liver surgery; LLS, laparoscopic liver surgery; RLS, robotic liver surgery; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; CRLM, colorectal cancer liver metastases; ASA score, American Society of Anaesthesiologists score.
Perioperative outcomes
Perioperative outcomes are depicted in Table 2.
Table 2
| Perioperative outcomes | OLS (n=124) | LLS (n=101) | RLS (n=47) | P value |
|---|---|---|---|---|
| Intraoperative outcomes | ||||
| Operative time (min) | 345 (345–345) | 329 (329–351) | 392 (319–489) | <0.001 |
| Blood loss (mL) | 450 (200–800) | 200 (50–425) | 200 (50–500) | <0.001 |
| Red blood cells transfusion | 18 (14.52) | 5 (4.95) | 6 (12.77) | 0.061 |
| Fresh frozen plasma transfusion | 7 (5.65) | 0 | 0 | 0.014 |
| R0 resection | 101 (81.45) | 98 (97.03) | 43 (91.49) | <0.001 |
| Conversion | – | 27 (26.73) | 8 (17.02) | 0.197 |
| Postoperative outcomes | ||||
| Morbidity | 64 (51.61) | 40 (39.60) | 22 (46.81) | 0.259 |
| Grade of complications (Clavien-Dindo) | ||||
| I grade | 10 (8.06) | 17 (16.83) | 5 (10.64) | 0.124 |
| II grade | 31 (25.00) | 18 (17.82) | 8 (17.02) | 0.324 |
| III–IV grade | 23 (18.55) | 5 (4.95) | 9 (19.15) | 0.006 |
| Liver-specific complications | 37 (29.84) | 15 (14.85) | 8 (17.02) | 0.018 |
| Liver failure | 4 (3.23) | 2 (1.98) | 0 | 0.433 |
| Ascites | 14 (11.29) | 2 (1.98) | 0 | 0.002 |
| Collection | 15 (12.10) | 9 (8.91) | 8 (17.02) | 0.359 |
| Bile leak | 11 (8.87) | 5 (4.95) | 1 (2.13) | 0.213 |
| General complications | 42 (33.87) | 27 (26.73) | 17 (36.17) | 0.397 |
| Haemorrhage | 20 (16.13) | 9 (8.91) | 4 (8.51) | 0.182 |
| Ileus | 5 (4.03) | 0 | 2 (4.26) | 0.120 |
| Chest infection | 6 (4.84) | 3 (2.97) | 1 (2.13) | 0.628 |
| Cardiovascular | 4 (3.23) | 2 (1.98) | 4 (8.51) | 0.137 |
| Urinary | 0 | 0 | 1 (2.13) | 0.091 |
| GI bleeding | 1 (0.81) | 2 (1.98) | 0 | 0.514 |
| Other | 10 (8.06) | 15 (14.85) | 6 (12.77) | 0.268 |
| Infectious complications | 27 (21.77) | 12 (11.88) | 6 (12.77) | 0.105 |
| Death | 1 (0.81) | 1 (0.99) | 0 (0.00) | <0.001 |
| Reoperation | 5 (4.03) | 4 (3.96) | 3 (6.38) | 0.770 |
| Total stay (days) | 12 (6–17) | 6 (4–11) | 5 (3–9) | <0.001 |
| Radiological imaging | 28 (22.58) | 22 (21.78) | 22 (46.81) | 0.612 |
| Antibiotic therapy | 28 (22.58) | 22 (21.78) | 13 (27.66) | 0.144 |
| Red blood cells transfusion | 42 (33.87) | 12 (11.88) | 6 (12.77) | 0.217 |
| Fresh frozen plasma transfusion | 18 (14.52) | 6 (5.94) | 5 (10.64) | 0.118 |
| Radio-guided or endoscopic procedures | 7 (5.65) | 5 (4.95) | 4 (8.51) | 0.686 |
The distribution of dichotomous categorical variables is expressed by n (%). For continuous variables, values are expressed as median (interquartile range). OLS, open liver surgery; LLS, laparoscopic liver surgery; RLS, robotic liver surgery; GI, gastrointestinal.
Patients in the RLS group had significantly longer operative time than those in the LLS and OLS groups (P<0.001). The two minimally invasive groups were associated with lower intraoperative blood loss as compared to the open (P<0.001). There were no differences between the three groups in RBC transfusion rate (P=0.061), however the OLS group received more FFP transfusions comparing with the other two (P=0.014). Significantly higher R0 resection rate was detected in the LLS and RLS groups than in the OLS group (97.03% for LLS vs. 91.49% for RLS vs. 81.45% for OLS, P<0.001). There were no significant differences observed between the two minimally invasive groups regarding the rate of conversion. However, patients in the RLS group showed a conversion rate of 17.02% compared to 26.73% in the laparoscopic group. Referring to the reason for conversion no patients have been converted for injury to adjacent organs. The main reason of conversion in both the minimally invasive groups has been “technical difficulties”. More patient in the laparoscopic group have been converted due to oncological radicality, but there is not a statistically significant difference between the two groups.
Although postoperative morbidity being similar comparing the three groups (P=0.259), the OLS group exhibited significantly higher severe complications when severity was taken into account (P=0.006). In particular, the LLS and the RLS groups experienced lower liver-specific complications (14.85% for LLS vs. 17.02% for RLS vs. 29.84% for OLS, P=0.018) and no ascites occurred in the RLS group (P=0.002). Moreover, the RLS group was associated with lower mortality rate (P<0.001) and shorter postoperative total stay (P<0.001). However, analysing the sub-population of patients with no postoperative complications, the median length of stay became similar among the three groups (4.5 days for the OLS group, 4 days for the two minimally invasive groups). No significant differences were found in terms of the need for radiological investigations, antibiotics and blood product transfusions among the groups.
Costs analysis
As reported in Table 3, for RLS the expenses related to surgical devices were significantly higher than the other two groups hence leading to globally higher intraoperative costs (+772.8% vs. OLS and +541.8% vs. LLS, P<0.001). In the context of intraoperative costs, it is important to note that histopathology exam, anaesthesia and sterilization costs remained consistent, regardless of the approach employed. The authors chose to include these costs in the analysis because they contribute to a more accurate estimate of the total intraoperative procedure costs, even though they do not have any impact in terms of differential analysis. On the other hand, a reduction in postoperative costs was seen for RLS (−56.0% vs. OLS and −29.4% vs. LLS, P<0.001) especially sustained by a marked reduction in hospital stays.
Table 3
| Cost drivers | OLS (n=124) (€) | LLS (n=101) (€) | RLS (n=47) (€) | Absolute difference (€) | Difference (%) | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | RLS vs. OLS | RLS vs. LLS | RLS vs. OLS | RLS vs. LLS | |||||||
| Intraoperative costs | ||||||||||||||||
| Medical staff | 1,189 | 348 | 1,172 | 326 | 1,148 | 378 | −41 | −24 | −3.45 | −1.99 | 0.799 | |||||
| Surgical devices | 443 | 4 | 602 | 134 | 3,864 | 229 | 3,421 | 3,262 | 772.84 | 541.80 | <0.001 | |||||
| Intraoperative transfusions | 128 | 374 | 30 | 135 | 44 | 116 | −84 | 14 | −65.58 | 45.63 | 0.029 | |||||
| Sterilization* | 198 | – | 198 | – | 198 | – | – | – | 0.00 | 0.00 | – | |||||
| Anaesthesia* | 117 | – | 117 | – | 117 | – | – | – | 0.00 | 0.00 | – | |||||
| Histopathological exam* | 75 | – | 75 | – | 75 | – | – | – | 0.00 | 0.00 | – | |||||
| Total | 2,150 | 663 | 2,194 | 422 | 5,446 | 538 | 3,296 | 3,252 | 153.34 | 148.23 | <0.001 | |||||
| Postoperative costs | ||||||||||||||||
| Hospital stays | 11,100 | 7,504 | 7,027 | 5,329 | 4,495 | 2,572 | −6,606 | −2,532 | −59.51 | −36.04 | <0.001 | |||||
| Intensive care unit stays | 108 | 415 | 33 | 235 | 250 | 605 | 142 | 217 | 131.48 | 657.58 | 0.026 | |||||
| Antibiotic therapies | 50 | 132 | 104 | 322 | 133 | 259 | 83 | 29 | 62.41 | 21.80 | 0.049 | |||||
| Postoperative transfusions | 416 | 1,014 | 96 | 349 | 180 | 555 | −236 | 84 | −131.11 | 46.67 | 0.005 | |||||
| Imaging & procedures | 136 | 735 | 89 | 310 | 133 | 236 | −2 | 45 | −1.60 | 50.50 | 0.583 | |||||
| Total | 11,810 | 8,487 | 7,349 | 5,921 | 5,191 | 3,262 | −6,619 | −2,158 | −56.05 | −29.36 | <0.001 | |||||
| Total cost | 13,960 | 8,765 | 9,543 | 6,125 | 10,637 | 3,409 | −3,323 | 1,094 | −23.80 | 11.47 | <0.001 | |||||
*, costs were identified by an extensive reviewed of the scientific literature. OLS, open liver surgery; LLS, laparoscopic liver surgery; RLS, robotic liver surgery.
Despite the comprehensive analysis, RLS had significantly higher costs for ICU stays compared to both OLS and LLS groups (+131.48% vs. OLS and +657.58% vs. LLS, P=0.026). Equally, the costs associated with antibiotic therapies were higher for RLS when compared with their counterparts (+62.4% vs. OLS and +21.8% vs. LLS, P=0.049). However, costs associated with postoperative blood products transfusions were lower for the RLS group if compared with the OLS group and higher if compared with the LLS group (−131.1% vs. OLS and +46.7% vs. LLS, P=0.005).
As a results, the balance between intra- and postoperative expenses turned into an overall cost of €13,960 for the OLS group, €9,543 for the LLS group and €10,637 for the RLS group (P<0.001).
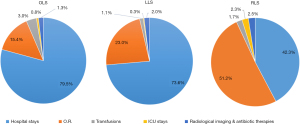
The analysis of cost distribution (Figure 1) reveals that in the OLS and LLS groups, a substantial portion of resources is absorbed by postoperative hospitalization costs (79.5% of total costs for the OLS group and 73.6% of total costs for the LLS group). In contrast, for the RLS group, the predominant resource absorption occurs during intraoperative costs (51.2% of total costs), although this is counterbalanced by a notable reduction in hospitalization-associated costs (42.3% of total costs).
The additional cost of conversion was +37.13% for the LLS group and +28.52% for the RLS group. However, this difference between the two minimally invasive groups did not reach statistical significance.
In addition, a detailed sub-analysis was conducted to compare the intra-, postoperative and total costs among patients who experience no postoperative complications (Table 4). By narrowing our focus to this specific subgroup, the authors found that the total costs associated with robotic surgery were 82.16% higher than those in the OLS group and 67.58% higher than those in the LLS group. Interestingly, intraoperative costs remained consistent with the main comparative analysis. Notably, the primary factor affecting postoperative costs—specifically the cost of hospital stay—did not exhibit substantial differences among the three groups in this sub-analysis (€3,105 for the OLS group, €3,131 for the LLS group, and €3,068 for the RLS group).
Table 4
| Cost drivers | OLS (n=16) (€) | LLS (n=34) (€) | RLS (n=33) (€) | Absolute difference (€) | Difference (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard deviation | Mean | Standard deviation | Mean | Standard deviation | RLS vs. OLS | RLS vs. LLS | RLS vs. OLS | RLS vs. LLS | |||||
| Intraoperative costs | ||||||||||||||
| Medical staff | 759 | 241 | 1,020 | 322 | 1,099 | 391 | 340 | 79 | 44.75 | 7.69 | ||||
| Surgical devices | 443 | 0 | 581 | 165 | 3,861 | 242 | 3,418 | 3,280 | 771.14 | 564.38 | ||||
| Intraoperative transfusions | – | – | 11 | 66 | 31 | 99 | 31 | 20 | NA | 171.31 | ||||
| Sterilization* | 198 | – | 198 | – | 198 | – | – | – | 0.00 | 0.00 | ||||
| Anaesthesia* | 117 | – | 117 | – | 117 | – | – | – | 0.00 | 0.00 | ||||
| Histopathological exam* | 75 | – | 75 | – | 75 | – | – | – | 0.00 | 0.00 | ||||
| Total | 1,592 | 241 | 2,002 | 324 | 5,381 | 563 | 3,789 | 3,379 | 237.90 | 168.64 | ||||
| Postoperative costs | ||||||||||||||
| Hospital stays | 3,105 | 1,293 | 3,131 | 1,199 | 3,068 | 1,160 | −37 | −63 | −1.19 | −2.01 | ||||
| Intensive care unit stays | – | – | 99 | 401 | 204 | 557 | 204 | 105 | NA | 106.06 | ||||
| Antibiotic therapies | – | – | 41 | 111 | 87 | 181 | 87 | 46 | NA | 112.05 | ||||
| Postoperative transfusions | 179 | 389 | 30 | 126 | 81 | 337 | −98 | 51 | −54.75 | 171.60 | ||||
| Imaging & procedures | 16 | 52 | 14 | 49 | 91 | 238 | 75 | 77 | 479.72 | 552.47 | ||||
| Total | 3,300 | 1,463 | 3,315 | 1,428 | 3,531 | 1,734 | 231 | 216 | 7.00 | 6.52 | ||||
| Total cost | 4,892 | 1,647 | 5,317 | 1,436 | 8,912 | 1,848 | 4,020 | 3,595 | 82.16 | 67.58 | ||||
*, costs were identified by an extensive reviewed of the scientific literature. OLS, open liver surgery; LLS, laparoscopic liver surgery; RLS, robotic liver surgery; NA, not applicable.
Discussion
RLS is gaining importance in the field of hepatobiliary surgery as a minimally invasive technique. To contribute to the literature in this field, the study aims to perform a rigorous economic analysis comparing open, laparoscopic, and robotic approaches using real-world evidence from two high-volume Italian centers and using a TD-ABC model, which provides a patient-specific cost estimation by considering various contributors to hospital costs. The economic analysis reveals that RLS has higher intraoperative expenses compared to laparoscopic and open approaches due to the surgical equipment costs. However, the RLS higher intraoperative costs are partially counterbalanced by postoperative savings sustained by a reduction of the hospital stay. A comprehensive breakdown of various cost components is provided, including personnel costs, disposable and reusable device costs, blood transfusion costs, antibiotic therapy costs, and postoperative investigation costs. Addressing the secondary endpoints, the results of the study show that RLS has a longer operative time compared to laparoscopic and open approaches, but the conversion rate is reduced in the RLS group, even though, it does not achieve statistical significance yet. RLS and laparoscopic approaches result in lower intraoperative blood loss compared to open surgery. The RLS and LLS groups also have a higher rate of R0 resections. Postoperative morbidity rates are similar among the three groups, but severe morbidity is significantly higher in the OLS group.
Despite the manageability and intuitiveness of the robotic platform, RLS accounts for less than 8% of all minimally invasive hepatectomies among the European countries (3). One of the most limiting factors is the uncertainty regarding the financial expenditure associated with robotic surgery corroborating that costs are an essential driving factor in health care.
Several studies have evaluated the cost effectiveness of robotic surgery in several fields (41-47). In urological surgery, when radical prostatectomy is concerned, the robotic approach seems to have higher costs that are not compensated by the reduced hospital stay. On the contrary, other procedures, such as cystectomy and partial nephrectomy, seem to be more cost-effective (42). In general surgery, some authors have been shown to lower overall cost by reducing complications, improving operative times, and cutting down on supplies (43-47). In more complex cases, such as hepatectomies, the reduction of complications, hospital stay, and readmissions could translate into a real cost benefit, even if the initial acquisition and intraoperative costs are higher. The largest monocentric series published by Sham et al. compared the costs of robotic hepatectomies (n=71) with open hepatectomies (n=88) conducted between 2011 and 2015. Although the robotic surgery group had higher peri-operative costs, the post-operative and total costs were lower than those of the control group underwent open hepatectomies (19). Similarly, in a retrospective study, Daskalaki et al. reported a significant overall lower rate of complications and costs in the robotic group than the subset of patients who underwent open liver resections (20). Aziz et al. utilized a nationwide database to assess long-term readmission rates for liver resections confirming that using robotics provides a financial benefit over open and LLS for patients underwent open and LLS (16). On the contrary Xu et al., investigating a single center series of patients that underwent liver resection for hilar cholangiocarcinoma, founded a hospital expenditure much higher in the robotic group compared to the traditional open surgery (21). Scientific evidence has been recently enriched by a meta-analysis published by Gavriilidis et al. Analysing 1,064 papers, the authors selected 79 articles with a population of 25,210 patients. The study results showed a cost saving of $1,197 by adopting the open technique and $759 by adopting the laparoscopic technique when compared to the robotic approach. Similarly, the laparoscopic approach showed a higher cost, approximately $426 more than the open approach (7). However, the procedure related costs did not statistically differ among the three groups. Previous studies have yielded conflicting results on the cost-effectiveness profile of RLS due to variations in cost calculation methods and the lack of consideration for the initial purchase costs of the robotic platform. The findings of the present study are a step forward with respect to the existent literature. Through a TD-ABC model, the cost of personnel and supply resources consumed by each patient and the time spent performing each activity have been extracted and validated by an expert opinion to perform a rigorous economic analysis that lay the foundation for future methodology, where reproducibility have to be an essential goal to evaluate cost trends.
Similarly to pure laparoscopy, RLS offers several clinical advantages over open surgery, including lower blood loss, decreased morbidity, shorter hospitalization, and faster recovery. RLS has shown efficacy in treating malignant diseases in terms of oncological adequacy while preserving the minimally invasive nature of the procedure. One of the major drawbacks of laparoscopic surgery is reduced dexterity and difficulty in performing complex tasks. Although laparoscopy is feasible, the caudal view and the linear shape of the instruments make surgeons more concerned about possible injuries in critical areas and any bleeding more challenging to control especially in proximity of major vessels or in case of vascular resections. Robotic technology addresses these limitations by providing instruments with high degrees of freedom, a stable 3D vision, tremor suppression algorithms, and enhanced ergonomics for the surgeon. This allows for more precise and complex manoeuvres, making RLS suitable for difficult liver resections such as postero-superior segments, major hepatectomies, and time-consuming interventions requiring extensive dissections or reconstructions. In a multicenter study by Cipriani et al., the authors concluded that robotics can favour the operative feasibility of difficult resections, possibly extending the indications for minimally invasive approaches for liver resections (2). Indeed, the use of robotic instrumentation may significantly resize complexity contributing to the large-scale diffusion of procedures with a high risk of injuries, such as performing an oncologically adequate lymph nodal dissection (48).
In a previous study by our group the outcomes of open vs. laparoscopic vs. robotic LND were specifically compared demonstrating a better performance of the robotic approach over laparoscopic approach (and both approaches over the open technique). The advantages of the robotic approach included image magnification, panoramic view, and the combination of specialized instrument, such as the Maryland bipolar forceps with empowered precision cutting and haemostatic ability and the prograsp forceps with a strong grip and wrist capabilities. These features allowed for effective exposure of the surgical field without obstructing the visual and operative field of the surgeon and provided a gentle dissection without incurring the risk of damage or breakage. Consequently, the use of robotics significantly reduced the time required for LND when compared to the laparoscopic approach (49).
Analysing the secondary endpoints, according with previous studies, the RLS group had a significantly longer operative time comparing with the other two groups, suggesting that robot docking and set-up still impact on the global length of the procedures (50). In addition, the fact that the two minimally invasive groups resulted in lower intraoperative blood loss supports the possibility of transection and haemostasis as accurate for RLS as for LLS even although an ultrasonic dissector specifically designed for robotics is not available yet. Although the production of a robotic ultrasonic dissector remains desirable this current absence is probably counterbalanced by the existing advanced robotic instruments that enable an accurate parenchymal dissection while preserving the vascular structures. Thereby, in our series, robotics show adequacy in the treatment of malignant diseases, and can offer advantages in achieving oncological radicality even presenting higher median size for the largest lesion. The data can be explained by the enhanced dexterity of robotics as it enables an accurate tracking of regulated transection planes, which is particularly challenging during laparoscopy (51). According with this result, even though no significant differences were recorded among the two minimally invasive groups in terms of rate of conversion, when analysing the reasons for conversion, RLS was associated with reduced conversions for oncologic radicality. Instead, the lower rate of R0 resections in the OLS group can be attributed to the fact that this group dealt with a higher proportion of technically challenging cases. These cases, characterized by a major tumor burden and the potential infiltration of vascular structures, are frequently addressed using an open approach due to the complexity of the surgery.
Furthermore, it is important to highlight that in the robotic group, a significantly higher percentage of patients had a history of previous abdominal surgery (78.72% vs. 56.44% for LLS vs. 50% for OLS, P=0.003). Notably, this did not lead to an increased conversion rate due to adhesions. This outcome underscores the efficacy of robotic instruments, likely facilitating effective adhesiolysis even in the most challenging cases.
Finally, there is a growing awareness of the fact, that the robotic learning curve in liver surgery is faster than the laparoscopic one. A steeper learning curve in comparison to LLS can be seen as a further advantage of the robotically assisted technique. Passing the learning curve helps to achieve a better cost-effectiveness, as operation time is decreasing and surgical outcomes are improving (52).
Intraoperative advantages directly translate into a favourable postoperative course. RLS in fact was associated with lower high-grade morbidity (grade 3–4 Clavien-Dindo), in particular ascites, and shorter postoperative hospital stay, significantly reducing costs needed for the postoperative management comparing with the other two groups. Thus, it is crucial to emphasize that among the statistically significant findings of our analysis, we have observed notable post-operative outcomes and, correspondingly, a reduction in post-operative costs related to RLS group. This observation potentially reinforces the robustness of the conducted cost analysis.
Nevertheless, it is essential to highlight that in the robotic group, cardiovascular accidents constituted 33.3% of severe complications. While the limited sample size prevents definitive conclusions, this data could be interpreted as a potential consequence of extended operative times, exposing patients to prolonged pneumoperitoneum (Table 5).
Table 5
| Severe complications (Clavien-Dindo 3–5) | OLS (n=23) | RLS (n=9) | P value |
|---|---|---|---|
| 3a | 11 (47.8) | 6 (66.7) | 0.337 |
| Bile leak | 5 (21.7) | 1 (11.1) | |
| Ascites | 1 (4.3) | 0 (0.0) | |
| Collection | 4 (17.4) | 2 (22.2) | |
| Bowel obstruction | 1 (4.3) | 0 (0.0) | |
| Cardiovascular disease | 0 (0.0) | 3 (33.3) | |
| 3b | 8 (34.8) | 3 (33.3) | 0.938 |
| Haemorrhage | 2 (8.7) | 1 (11.1) | |
| Bowel obstruction | 2 (8.7) | 2 (22.2) | |
| Pneumothorax | 1 (4.3) | 0 (0.0) | |
| Choleperitoneum | 3 (13.0) | 0 (0.0) | |
| 4a | 2 (8.7) | 0 (0.0) | 0.361 |
| Liver failure | 1 (4.3) | 0 (0.0) | |
| Respiratory failure | 1 (4.3) | 0 (0.0) | |
| 4b | 1 (4.3) | 0 (0.0) | 0.525 |
| Multiorgan failure | 1 (4.3) | 0 (0.0) | |
| 5 | 1 (4.3) | 0 (0.0) | 0.525 |
| Death | 1 (4.3) | 0 (0.0) |
The distribution of dichotomous categorical variables is expressed by n (%). OLS, open liver surgery; RLS, robotic liver surgery.
In the economic sub-analysis of patients without postoperative complications, total costs for robotic surgery were notably higher than those for open and laparoscopic approaches. This increase was primarily due to the variations in hospital stay expenses, which, in this case, were relatively similar among the three groups. This economic finding aligns with the clinical observation that the median LOS was 4.5 days for the OLS group, 4 days for the LLS group, and 4 days for the RLS group. Consequently, the initial hypothesis is further supported, indicating that the robotic approach is particularly advantageous for high-risk hepatectomies prone to postoperative complications, such as complex liver resections.
Regarding the ICU stay, it is worth to mention that the results in terms of absorption of direct healthcare costs for the robotic approach could be overestimated since authors considering a cautious management of the initial cohort. On the other hand, in order to give an explanation on the higher usage and costs of antibiotic treatment in the RLS group, it is relevant to reference that in this group, patients who underwent hilar LND are more prominently represented, and these cases more frequently necessitate post-operative antibiotic treatment.
Concerning patient selection, the RLS group presented a higher proportion of cases with benign diseases. It is important to clarify that a significant subset of these patients was affected by benign diseases of the intrahepatic biliary tract (i.e., inflammatory stenosis and hepatolithiasis). These conditions frequently required complex parenchymal dissection due to the distortion of intrahepatic ducts and the vascular changes resulting from the inflammatory extension of cholangitis (53). Given this challenging anatomical situation, the decision to opt for a robotic approach is related to enhanced dexterity ad precise cutting capabilities, crucial for effective manoeuvres within an inflammation affected environment. The ability to perform delicate microsuturing with robotic assistance further supports the suitability of robotic approach in such cases.
In order to provide a real-life picture of allocation to each available approach, a simple comparison without propensity score analysis was chosen as the best design to fulfil study endpoints. Provided the specific indications and contraindications for each approach—including the complexity of the disease, the presence of severe comorbidities, anatomical constraints and the need for extensive liver resections—a matching according to preoperative variables would not have been appropriate and not informative as there were substantial differences between patients who underwent minimally invasive procedures and those who received open surgery. Hence the absence of a propensity score analysis in this scenario reflects the inherent complexity and variability of patient cases, where individualized treatment plans are essential to ensure the best possible outcomes.
However, the study is not exempt from limitations. The sample size was is still relatively limited, which could affect the statistical power of the results. With a small sample, the study may not capture the full range of variations and potential outcomes, reducing the reliability of the findings. The second limitation relates to the economic analysis conducted in the study. The costs were derived from literature sources, including the acquisition costs of the robotic platform and the costs of dissectors based on average prices obtained from regional Italian tenders. Relying solely on literature sources may introduce potential inaccuracies and regional variations in the cost estimates. However, to enhance the generalizability of the results, the study utilized existing literature sources instead of data from administrative databases. In fact, these sources provided a broader perspective and allowed for the consideration of various settings beyond the specific centers involved in the study. Another relevant limitation of the study is the failure to consider the long-term amortization of the robotic platform cost, which could have a significant impact on the cost-effectiveness profile considering a longer time horizon. This aspect was not addressed because reimbursement diagnosis-related groups (DRGs) vary across regions, and it would have been necessary to take into account also all procedures performed using the robotic platform by all the other surgical units. By not accounting for the long-term cost implications and considering the broader range of procedures conducted with the robotic platform, the study may underestimate or overlook the true cost-effectiveness value of the robotic approach. Future research endeavors should delve into assessing long-term patient outcomes, refining cost-saving measures, and exploring the implications of new robotic systems to enhance the overall effectiveness and applicability of RLS in clinical practice.
Conclusions
This study emphasizes the potential advantages of RLS in complex resections and underscores the need for a thorough economic analysis for cost-effectiveness, providing real-world evidence and detailed economic insights using a TD-ABC model. Despite RLS incurring higher total intraoperative costs, including ICU stays and surgical devices, when compared to OLS and LLS groups, it demonstrated lower total postoperative costs, resulting in an overall cost of €10,637, compared to €13,960 for OLS. Anticipated reductions in acquisition costs with the introduction of new robotic systems and increased market competition make robotic surgery a favoured minimally invasive technique. As adoption expands, proficiency among surgeons and healthcare providers is expected to lead to streamlined procedures, shorter operative times, and improved patient outcomes, contributing to lowered overall costs associated with RLS. Over time, standardization and proficiency in using robotic systems have the potential to optimize workflow, reduce complications, and yield cost savings. However, it is essential to consider factors such as maintenance, upgrades, and ongoing training when evaluating the overall cost-effectiveness of robotic technology. A comprehensive long-term cost analysis should encompass these elements to grasp the economic implications of widespread adoption in minimally invasive techniques.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-407/dss
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-407/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-407/coif). L.A. serves as an unpaid editorial board member of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study adheres to the principles outlined in the Declaration of Helsinki (as revised in 2013). The study population derived from the Italian “I GoMILS” registry. Ethical approval for the registry was granted by the Ethics Committee of the promoting center and shared among the participants (IGOMILS-OSR of March 6, 2014—available at: https://www.cr-technology.com/igomils/eclinical/website/documents.aspx). Written consent from subjects was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432-44. [Crossref] [PubMed]
- Cipriani F, Fiorentini G, Magistri P, et al. Pure laparoscopic versus robotic liver resections: Multicentric propensity score-based analysis with stratification according to difficulty scores. J Hepatobiliary Pancreat Sci 2022;29:1108-23. [Crossref] [PubMed]
- Zwart MJW, Görgec B, Arabiyat A, et al. Pan-European survey on the implementation of robotic and laparoscopic minimally invasive liver surgery. HPB (Oxford) 2022;24:322-31. [Crossref] [PubMed]
- Ciria R, Berardi G, Alconchel F, et al. The impact of robotics in liver surgery: A worldwide systematic review and short-term outcomes meta-analysis on 2,728 cases. J Hepatobiliary Pancreat Sci 2022;29:181-97. [Crossref] [PubMed]
- Rahimli M, Perrakis A, Andric M, et al. Does Robotic Liver Surgery Enhance R0 Results in Liver Malignancies during Minimally Invasive Liver Surgery?-A Systematic Review and Meta-Analysis. Cancers (Basel) 2022;14:3360. [Crossref] [PubMed]
- Mangano A, Valle V, Masrur MA, et al. Robotic liver surgery: literature review and future perspectives. Minerva Surg 2021;76:105-15. [Crossref] [PubMed]
- Gavriilidis P, Roberts KJ, Aldrighetti L, et al. A comparison between robotic, laparoscopic and open hepatectomy: A systematic review and network meta-analysis. Eur J Surg Oncol 2020;46:1214-24. [Crossref] [PubMed]
- Moorthy K, Munz Y, Dosis A, et al. Dexterity enhancement with robotic surgery. Surg Endosc 2004;18:790-5. [Crossref] [PubMed]
- Mucksavage P, Kerbl DC, Lee JY. The da Vinci(®) Surgical System overcomes innate hand dominance. J Endourol 2011;25:1385-8. [Crossref] [PubMed]
- Wu CY, Chen PD, Chou WH, et al. Is robotic hepatectomy cost-effective? In view of patient-reported outcomes. Asian J Surg 2019;42:543-50. [Crossref] [PubMed]
- Patriti A, Cipriani F, Ratti F, et al. Robot-assisted versus open liver resection in the right posterior section. JSLS 2014;18:e2014.00040.
- Di Benedetto F, Petrowsky H, Magistri P, et al. Robotic liver resection: Hurdles and beyond. Int J Surg 2020;82S:155-62. [Crossref] [PubMed]
- Stewart C, Wong P, Warner S, et al. Robotic minor hepatectomy: optimizing outcomes and cost of care. HPB (Oxford) 2021;23:700-6. [Crossref] [PubMed]
- Masetti M, Fallani G, Ratti F, et al. Minimally invasive treatment of colorectal liver metastases: does robotic surgery provide any technical advantages over laparoscopy? A multicenter analysis from the IGoMILS (Italian Group of Minimally Invasive Liver Surgery) registry. Updates Surg 2022;74:535-45. [Crossref] [PubMed]
- Miller HP, Hakim A, Kellish A, et al. Cost-Benefit Analysis of Robotic vs. Laparoscopic Hepatectomy: A Propensity-Matched Retrospective Cohort Study of American College of Surgeons National Surgical Quality Improvement Program Database. Am Surg 2022;88:2886-92. [Crossref] [PubMed]
- Aziz H, Hanna K, Lashkari N, et al. Hospitalization Costs and Outcomes of Open, Laparoscopic, and Robotic Liver Resections. Am Surg 2022;88:2331-7. [Crossref] [PubMed]
- Cortolillo N, Patel C, Parreco J, et al. Nationwide outcomes and costs of laparoscopic and robotic vs. open hepatectomy. J Robot Surg 2019;13:557-65. [Crossref] [PubMed]
- Hu M, Liu Y, Li C, et al. Robotic versus laparoscopic liver resection in complex cases of left lateral sectionectomy. Int J Surg 2019;67:54-60. [Crossref] [PubMed]
- Sham JG, Richards MK, Seo YD, et al. Efficacy and cost of robotic hepatectomy: is the robot cost-prohibitive? J Robot Surg 2016;10:307-13. [Crossref] [PubMed]
- Daskalaki D, Gonzalez-Heredia R, Brown M, et al. Financial Impact of the Robotic Approach in Liver Surgery: A Comparative Study of Clinical Outcomes and Costs Between the Robotic and Open Technique in a Single Institution. J Laparoendosc Adv Surg Tech A 2017;27:375-82. [Crossref] [PubMed]
- Xu Y, Wang H, Ji W, et al. Robotic radical resection for hilar cholangiocarcinoma: perioperative and long-term outcomes of an initial series. Surg Endosc 2016;30:3060-70. [Crossref] [PubMed]
- Strasberg SM. Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 2005;12:351-5. [Crossref] [PubMed]
- Le Foie, Couinaud C. Etudes anatomiques et chirurgicales. Paris: Mason, 1957:284-9.
- Molinari M, El-Tawil K, Swaid F, et al. Patients' treatment preferences for potentially resectable tumors of the head of the pancreas. HPB (Oxford) 2020;22:265-74. [Crossref] [PubMed]
- Aldrighetti L, Catena M, Ratti F. Maximizing Performance in Complex Minimally Invasive Surgery of the Liver: the RoboLap Approach. J Gastrointest Surg 2022;26:1811-3. [Crossref] [PubMed]
- Ratti F, Cipriani F, Lee Y, et al. Minimally-invasive Right Hepatectomy for Perihilar Cholangiocarcinoma. Chirurgia (Bucur) 2022;117:110-3. [Crossref] [PubMed]
- Ratti F, Fiorentini G, Cipriani F, et al. Technical Insights on Laparoscopic Left and Right Hepatectomy for Perihilar Cholangiocarcinoma. Ann Surg Oncol 2020;27:5191-2. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ariotti R, et al. Laparoscopic major hepatectomies: current trends and indications. A comparison with the open technique. Updates Surg 2015;67:157-67. [Crossref] [PubMed]
- Bolliger M, Kroehnert JA, Molineus F, et al. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur Surg 2018;50:256-61. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg 2009;144:46-51. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Hamady ZZ, Cameron IC, Wyatt J, et al. Resection margin in patients undergoing hepatectomy for colorectal liver metastasis: a critical appraisal of the 1cm rule. Eur J Surg Oncol 2006;32:557-63. [Crossref] [PubMed]
- Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev 2004;82:131-8, 150. [PubMed]
- Najjar PA, Strickland M, Kaplan RS. Time-Driven Activity-Based Costing for Surgical Episodes. JAMA Surg 2017;152:96-7. [Crossref] [PubMed]
- Goldberg MJ, Kosinski L. Activity-based costing and management in a hospital-based GI unit. Clin Gastroenterol Hepatol 2011;9:947-949.e1. [Crossref] [PubMed]
- Tedesco G, Faggiano FC, Leo E, et al. A comparative cost analysis of robotic-assisted surgery versus laparoscopic surgery and open surgery: the necessity of investing knowledgeably. Surg Endosc 2016;30:5044-51. [Crossref] [PubMed]
- Dubray Q, Laroche S, Tribillon E, et al. Analysis of economic impact of laparoscopic liver resection according to surgical difficulty. Surg Endosc 2021;35:1006-13. [Crossref] [PubMed]
- Tan SS, Bakker J, Hoogendoorn ME, et al. Direct cost analysis of intensive care unit stay in four European countries: applying a standardized costing methodology. Value Health 2012;15:81-6. [Crossref] [PubMed]
- Ministero dell’Economia e delle Finanze Commissione Tecnica per la Finanza Pubblica. Libro verde sulla spesa pubblica. Available online: https://www.mef.gov.it/ministero/commissioni/ctfp/documenti/Libro_verde_spesa_pubblica.pdf
- Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. [Crossref] [PubMed]
- Ahmed K, Ibrahim A, Wang TT, et al. Assessing the cost effectiveness of robotics in urological surgery - a systematic review. BJU Int 2012;110:1544-56. [Crossref] [PubMed]
- Byrn JC, Hrabe JE, Charlton ME. An initial experience with 85 consecutive robotic-assisted rectal dissections: improved operating times and lower costs with experience. Surg Endosc 2014;28:3101-7. [Crossref] [PubMed]
- Bedeir K, Mann A, Youssef Y. Robotic single-site versus laparoscopic cholecystectomy: Which is cheaper? A cost report and analysis. Surg Endosc 2016;30:267-72. [Crossref] [PubMed]
- Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg 2012;22:52-61. [Crossref] [PubMed]
- Wright JD, Ananth CV, Tergas AI, et al. An economic analysis of robotically assisted hysterectomy. Obstet Gynecol 2014;123:1038-48. [Crossref] [PubMed]
- Anderson JE, Chang DC, Parsons JK, et al. The first national examination of outcomes and trends in robotic surgery in the United States. J Am Coll Surg 2012;215:107-14; discussion 114-6. [Crossref] [PubMed]
- Fiorentini G, Ratti F, Cipriani F, et al. Challenges and Technical Innovations for an Effective Laparoscopic Lymphadenectomy in Liver Malignancies. J Laparoendosc Adv Surg Tech A 2019;29:72-5. [Crossref] [PubMed]
- Ratti F, Cipriani F, Ingallinella S, et al. Robotic Approach for Lymphadenectomy in Biliary Tumors: The Missing Ring Between the Benefits of Laparoscopic and Reproducibility of Open Approach? Ann Surg 2023;278:e780-8. [Crossref] [PubMed]
- Kamarajah SK, Bundred J, Manas D, et al. Robotic versus conventional laparoscopic liver resections: A systematic review and meta-analysis. Scand J Surg 2021;110:290-300. [Crossref] [PubMed]
- Cipriani F, Shelat VG, Rawashdeh M, et al. Laparoscopic Parenchymal-Sparing Resections for Nonperipheral Liver Lesions, the Diamond Technique: Technical Aspects, Clinical Outcomes, and Oncologic Efficiency. J Am Coll Surg 2015;221:265-72. [Crossref] [PubMed]
- Spiegelberg J, Iken T, Diener MK, et al. Robotic-Assisted Surgery for Primary Hepatobiliary Tumors-Possibilities and Limitations. Cancers (Basel) 2022;14:265. [Crossref] [PubMed]
- Nguyen Canh H, Harada K. Adult bile duct strictures: differentiating benign biliary stenosis from cholangiocarcinoma. Med Mol Morphol 2016;49:189-202. [Crossref] [PubMed]


