
Treatment of portal vein thrombosis in cirrhosis with anticoagulation—more than meets the eye?
Portal vein thrombosis (PVT) is a common complication in patients with advanced chronic liver disease (i.e., cirrhosis). In contrast to other thrombotic diseases, PVT in patients with cirrhosis is frequently asymptomatic and discovered incidentally during routine imaging procedures. There is ongoing debate on whether all patients with PVT require treatment as it is unclear whether PVT is a relatively innocent bystander or whether PVT worsens disease progression (1). Treatment may be required in patients with >50% occlusion of the portal vein who are transplant candidates to avoid thrombosis progression that may hinder a future liver transplantation or cause progression of portal hypertension (2). Current treatment consists of therapeutic anticoagulation with vitamin K antagonists, heparins, or direct oral anticoagulants. Although anticoagulant therapy for cirrhotic PVT is relatively safe, the efficacy is modest, with meta-analyses demonstrating recanalization of the portal vein in ~30–40% of patients without anticoagulation, and ~60–70% with anticoagulation (3,4).
The modest effect of anticoagulant therapy for PVT in patients with cirrhosis may relate to the pathogenesis of the disease. While thrombotic events in other venous vascular beds, notably deep vein thrombosis (DVT) of the leg and pulmonary embolism (PE) are clearly caused by intravascular clot formation, the role of clot formation in driving PVT is less clear. Firstly, while hypercoagulable factors such as carriership of FVleiden or non-O blood group are clear risk factors for development of DVT or PE, hypercoagulability appears to play a much more modest role (if any) in development of PVT (5,6). Secondly, the composition of a portal vein thrombus is completely different from that of a thrombus in a patient with DVT or PE. While a thrombus in a patient with DVT or PE consists of fibrin, platelets, and red blood cells (7), a portal vein thrombus often completely lacks these classical clot components (8). The majority of portal vein thrombi consist of a thickened portal vein wall intima as we demonstrated in a recent study in which we examined portal vein thrombi retrieved during liver transplantation procedures (8) (see Figure 1). These observations argue against PVT as a true thrombotic disease, and we proposed that portal vein obstruction or portal vein stenosis may be a more appropriate term for what is now classified as cirrhotic PVT. Interestingly, mild portal vein intima hyperplasia is also observed in patients with cirrhosis but without PVT (Figure 1), suggesting that exacerbation of portal vein intimal hyperplasia underlies development of PVT. It is unknown how portal vein intimal hyperplasia develops in patients with cirrhosis, but a likely mechanism relates to changes in portal vein flow as a consequence of portal hypertension, as changes in flow are known to cause intimal hyperplasia in other vascular beds, such as coronary artery bypass grafting using autologous veins (10). Such a mechanism aligns with the observation that alterations in portal flow, and not markers of hypercoagulability or inflammation, predict future development of PVT in patients with cirrhosis (5).
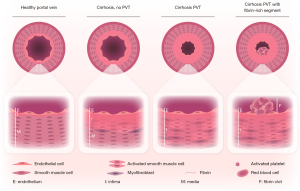
Although it has been previously established that treatment with anticoagulant drugs helps to recanalize the portal vein in some patients with PVT, it has been debated whether successful recanalization is beneficial for the patient beyond facilitating liver transplantation. Whereas some studies demonstrated that PVT results in accelerated progression of disease (11), other studies suggested that PVT is merely a reflection of severity of disease with no effect on progression of disease (12). In a recent publication, Guerrero and coworkers reported an individual patient data meta-analysis of studies that compared patients that received anticoagulant therapy with the aim to treat PVT with patients with PVT that did not receive anticoagulation (3). This study showed a clear and clinically significant survival benefit of anticoagulant treatment (a 36% relative risk reduction in all-cause mortality). Importantly and surprisingly, the survival benefit of anticoagulation was independent of portal vein recanalization. Also in this study, the effect of anticoagulation on portal vein recanalization was modest (with 58% of patients recanalizing with anticoagulation, while 33% of patients recanalize without anticoagulation). These results suggest that anticoagulant treatment has PVT-independent effects on outcome in patients with cirrhosis. This in turn suggests that the aim of anticoagulant treatment of patients with cirrhotic PVT should not primarily be portal vein recanalization, but rather that anticoagulant treatment should be considered with the aim to improve the general condition of the patient.
The observation that anticoagulants are beneficial for patients with cirrhosis is not new. Extensive experimental studies have demonstrated that anticoagulant reduce disease progression in models of acute and chronic liver injury by preventing intrahepatic microthrombosis and by reducing activation of intrahepatic cells (notably stellate cells) by coagulation proteases such as thrombin and factor Xa (13). In addition, a small randomized controlled trial has demonstrated that anticoagulant treatment of patients with cirrhosis, but without PVT, reduces the risk for decompensation and death (14). Furthermore, anticoagulation of patients with cirrhosis and atrial fibrillation is associated with a survival benefit (15). These observations thus strongly suggest to consider anticoagulation for all patients with PVT. Current clinical guidance documents indicate that there are situations in which anticoagulation for asymptomatic cirrhotic PVT should be considered (e.g., recent occlusive or partially occlusive thrombosis with >50% occlusion), but are generally conservative particularly when the patient is not a transplant candidate. The study of Guererro suggests that a more liberal approach to anticoagulant therapy for cirrhotic PVT may be more appropriate.
The downside of anticoagulant therapy is an increased bleeding risk. Patients with cirrhosis may acquire major alterations in their hemostatic system, which may make them more susceptible to anticoagulation-related bleeding complications (16). The decision to initiate or continue anticoagulation should thus be carefully weighed against the bleeding risk. Importantly, anticoagulation may paradoxically reduce bleeding risk in patients with cirrhosis, as anticoagulation may reduce portal hypertension, which in turn reduces the risk for portal hypertension-associated bleeding (e.g., variceal bleeding) (4). The altered hemostatic parameters in patients with cirrhosis complicate an adequate assessment of bleeding risk, and decisions to initiate anticoagulation in patients with cirrhosis that are frail and have co-morbidities may require a multidisciplinary discussion.
Although the study of Guerrero and coworkers may be a game-changer as it shows that a simple and affordable pharmacological intervention is associated with a substantial survival benefit, some limitations of the study that were not discussed by the authors themselves deserve attention. First, the age of the patients was relatively low, which may suggest that many patients were candidates for liver transplantation, and therefore had an indication for anticoagulant therapy. Second, the study assessed patients with both occlusive and non-occlusive PVT, without a clear description of characteristics of the partial thrombi. Third, patients were treated with either vitamin K antagonists or heparins, but no patients receiving direct oral anticoagulants were studied. Thus, further studies, preferable large well-designed randomized trials, are required to definitively establish the role of anticoagulants in patients with cirrhotic PVT.
An unsolved issue is how to improve recanalization rates of patients with PVT that are transplant candidates. A patent portal vein at the time of liver transplantation simplifies the surgical procedure, and may improve graft survival. A substantial proportion of patients does not adequately respond to anticoagulant therapy, which is understandable in light of the observation that may portal vein thrombi are devoid of fibrin (8). Studies on alternative treatment strategies for cirrhotic PVT that are directed at decreasing portal vein intimal hyperplasia are required. Even better, strategies to avoid development of PVT should be better explored. Interestingly, anticoagulant treatment has been demonstrated to substantially decrease PVT development (13), which may relate to the role of the coagulation system in development of intimal hyperplasia (8).
In summary, there have been major recent advances in our understanding of the pathogenesis and treatment of PVT in patients with cirrhosis. It appears that anticoagulant treatment is remarkably beneficial for these patients, but this benefit seems independent of thrombus resolution. Rather, PVT-independent mechanisms such as prevention or treatment of intrahepatic microthrombosis may be central in the success of anticoagulant treatment in this setting. Studies specifically aimed at exploiting these PVT-independent beneficial effects of anticoagulant therapy need to be developed for patients with various stages of advanced chronic liver disease. In addition, alternative strategies for portal vein recanalization in patients with cirrhosis may be required.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-669/coif). The author has no conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Faccia M, Ainora ME, Ponziani FR, et al. Portal vein thrombosis in cirrhosis: Why a well-known complication is still matter of debate. World J Gastroenterol 2019;25:4437-51. [Crossref] [PubMed]
- Northup PG, Garcia-Pagan JC, Garcia-Tsao G, et al. Vascular Liver Disorders, Portal Vein Thrombosis, and Procedural Bleeding in Patients With Liver Disease: 2020 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021;73:366-413. [Crossref] [PubMed]
- Guerrero A, Campo LD, Piscaglia F, et al. Anticoagulation improves survival in patients with cirrhosis and portal vein thrombosis: The IMPORTAL competing-risk meta-analysis. J Hepatol 2023;79:69-78. [Crossref] [PubMed]
- Loffredo L, Pastori D, Farcomeni A, et al. Effects of Anticoagulants in Patients With Cirrhosis and Portal Vein Thrombosis: A Systematic Review and Meta-analysis. Gastroenterology 2017;153:480-487.e1. [Crossref] [PubMed]
- Turon F, Driever EG, Baiges A, et al. Predicting portal thrombosis in cirrhosis: A prospective study of clinical, ultrasonographic and hemostatic factors. J Hepatol 2021;75:1367-76. [Crossref] [PubMed]
- Scheiner B, Northup PG, Gruber AB, et al. The impact of ABO blood type on the prevalence of portal vein thrombosis in patients with advanced chronic liver disease. Liver Int 2020;40:1415-26. [Crossref] [PubMed]
- Chernysh IN, Nagaswami C, Kosolapova S, et al. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep 2020;10:5112. [Crossref] [PubMed]
- Driever EG, von Meijenfeldt FA, Adelmeijer J, et al. Nonmalignant portal vein thrombi in patients with cirrhosis consist of intimal fibrosis with or without a fibrin-rich thrombus. Hepatology 2022;75:898-911. [Crossref] [PubMed]
- Lisman T. Bleeding and Thrombosis in Patients With Cirrhosis: What's New?. Hemasphere 2023;7:e886. [Crossref] [PubMed]
- Harskamp RE, Lopes RD, Baisden CE, et al. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg 2013;257:824-33. [Crossref] [PubMed]
- Englesbe MJ, Kubus J, Muhammad W, et al. Portal vein thrombosis and survival in patients with cirrhosis. Liver Transpl 2010;16:83-90. [Crossref] [PubMed]
- Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology 2015;61:660-7. [Crossref] [PubMed]
- Kopec AK, Joshi N, Luyendyk JP. Role of hemostatic factors in hepatic injury and disease: animal models de-liver. J Thromb Haemost 2016;14:1337-49. [Crossref] [PubMed]
- Villa E, Cammà C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012;143:1253-1260.e4. [Crossref] [PubMed]
- Serper M, Weinberg EM, Cohen JB, et al. Mortality and Hepatic Decompensation in Patients With Cirrhosis and Atrial Fibrillation Treated With Anticoagulation. Hepatology 2021;73:219-32. [Crossref] [PubMed]
- van den Boom BP, Lisman T. Pathophysiology and management of bleeding and thrombosis in patients with liver disease. Int J Lab Hematol 2022;44:79-88. [Crossref] [PubMed]


