
Exercise in the management of metabolic dysfunction-associated fatty liver disease—is more the answer, or is it just one piece of the puzzle?
Metabolic dysfunction-associated fatty liver disease (MAFLD) is the liver manifestation of metabolic syndrome, a rapidly growing clinical and public health issue mirrored by epidemic rates of obesity, type 2 diabetes mellitus, hypertension and cardiovascular disease (CVD). Besides MAFLD, nomenclature used to capture fatty liver disease not primarily due to alcohol excess include non-alcoholic fatty liver disease (NAFLD) and metabolic dysfunction-associated steatotic liver disease (MASLD). Regardless of the terminology used, these diagnoses describe the most common cause of chronic liver disease globally. Accordingly, MAFLD is poised to become the leading cause of hepatocellular carcinoma (HCC) and end-stage liver disease requiring liver transplantation around the world (1,2). In addition to this, CVD and extra-hepatic cancers are the two main causes of death in the MAFLD population. Thus, MAFLD poses a severe clinical burden which is projected to worsen in the future and intervention is urgently needed.
Weight loss through lifestyle modification remains the first-line treatment of MAFLD. It is effective at improving liver enzymes and histology (reducing/resolving steatosis and steatohepatitis and even regressing fibrosis) in a dose-dependent manner with most benefit seen in patients who lose >7–10% of their bodyweight (3). Furthermore, sustained weight loss also leads to meaningful benefits in components of the metabolic syndrome. Although there are many ways to achieve weight loss, exercise clearly plays a key role. In a recent review, Keating et al. on behalf of Exercise and Sports Science Australia, present evidence-based and consensus recommendations regarding the role of exercise in the management of MAFLD (4). Although a formalised (e.g., Delphi) method was not used, consensus was reached through an iterative process involving the multi-disciplinary authorship team and decisions on translating evidence-based guidelines into clinical practice were based on authors’ professional experiences. Nonetheless, this position statement is welcomed for two main reasons. First, clinicians are often ill-equipped and ill-informed regarding prescribing exercise for MAFLD (and other conditions) and this review may help to upskill them (5). Second, it provides a good reference on the clinical aspects of MAFLD for exercise professionals (exercise physiologists, physiotherapists, trainers, etc.) as they become increasingly important in its multi-disciplinary management (discussed further below).
Currently, the strongest evidence supports that 150–240 minutes per week of at least moderate-intensity aerobic exercise can lead to modest absolute reductions in hepatic steatosis by 2–4% (4). Even as little as 135 minutes per week (19 minutes per day) has been shown to be effective. Although high-intensity interval training is likely to yield similar results, its limited evidence prevented the authors from making a firm recommendation. Conversely, there is more uncertainty regarding the role of resistance training alone due to mixed data on hepatic steatosis reduction. As such this and other reviews have recommended that resistance training can be complementary to aerobic exercise but not a replacement (6). Of note, it appears that beyond the minimum threshold of 135 minutes of moderate-intensity exercise per week, further increases in exercise volume (minutes) or intensity provide no additional benefit on reducing hepatic steatosis. Interestingly, the benefit of exercise on hepatic steatosis can also occur with minimal or no weight loss (7).
Importantly, in contrast to weight loss, there are limited high quality data supporting the efficacy of exercise for improving histological features of MAFLD beyond steatosis. These other histological endpoints (i.e., inflammation and fibrosis) have greater influence on the development of clinical events such as liver complications (6). Indeed, the severity of steatosis as measured non-invasively by controlled attenuation parameter on transient elastography has not been shown to predict liver-related events (HCC or decompensation), non-HCC cancer or cardiovascular events in patients with NAFLD (8). One explanation for this may be that improvement of liver inflammation and/or fibrosis may be more dependent on weight loss while exercise alone (without dietary modification) only has a modest effect on this (~2–3 kg decrease in bodyweight and ~0.78 kg/m2 decrease in body mass index) (4). It should be remembered that common to both exercise and weight loss are observed improvements in general fitness and wellbeing, and cardiovascular parameters (particularly dyslipidaemia) which may prevent the development of diabetes mellitus and CVD (9).
The above highlights that exercise alone (and simply more of it) may not be sufficient to alter the natural history of MAFLD, a multi-factorial disease. Instead, dietary, pharmacological and even surgical interventions as well as lifestyle modifications are necessary. This is best delivered through a multi-disciplinary team approach as suggested by Keating et al. and many others (4,9,10), similar to the management of HCC (Figure 1). For instance, the combination of exercise with dietary modification has been consistently shown to be synergistic and associated with greater weight loss and reduction in hepatic steatosis compared to exercise alone (4,10). Thus, the involvement of a dietitian in addition to an exercise professional is ideal. Other facets of MAFLD that need addressing include smoking cessation, minimising alcohol consumption, aggressive management of metabolic comorbidities (type 2 diabetes, hypertension, dyslipidaemia) and cancer screening via a primary care physician and/or medical specialist(s) (6). Specific to exercise, complications of obesity (e.g., obstructive sleep apnoea, osteoarthritis, mental illness) which may limit a patient’s ability to exercise also require concurrent treatment. Beyond lifestyle modification, there will likely be approved effective pharmacotherapies for MAFLD in the near future and indeed some (e.g., glucagon-like peptide 1 agonists) have already been used off-label for their weight loss properties. Bariatric surgery for select patients with non-cirrhotic obese MAFLD is effective at reducing weight, resolving steatohepatitis and regressing fibrosis (11). However, pharmacotherapy and bariatric surgery are not appropriate or beneficial for all patients with MAFLD and are reserved for those who have already tried but not responded to lifestyle modification. Even if these MAFLD treatments have been effective, ongoing exercise and dietary modification may help in maintaining weight loss and other improvements (12), again highlighting the need for multi-disciplinary care. At the centre of any multi-disciplinary care model is the patient as illustrated in Keating et al.’s suggested approach (4). Indeed, patients with MAFLD are ultimately the ones who need to be motivated and engaged with their (realistic and achievable) prescribed interventions. As such, a pragmatic individualised approach which takes into account each patient’s preferences and clinical situation has been recommended by most MAFLD guidelines (6,10).
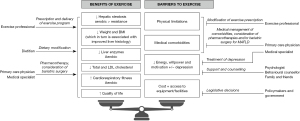
Several points mentioned by Keating et al. deserve to be highlighted. First, a barrier to exercise may be patients’ lack of education on how to exercise and the importance of managing MAFLD. Indeed, the members of the multi-disciplinary team all play a role in providing this education. Additionally, the European Association for the Study of the Liver has recently published the first ever patient guidelines on living with MAFLD, an accessible lay summary intended for educating patients and the general public (9). Patient representatives took part in the development of these patient guidelines. Since patients are at the centre of the multi-disciplinary approach to MAFLD management, patient representatives should similarly be involved in future position statements and guidelines on exercise. Second, the majority of evidence on exercise is on early stage MAFLD, with limited data from patients with advanced liver disease and cirrhosis. Exercise has been shown to be safe and beneficial in cirrhosis of other aetiologies (13). However, it is likely that patients with MAFLD cirrhosis will have physical limitations which restrict their tolerance of prescribed exercise. Therefore, it has been recommended that strenuous activities should be avoided especially in those with decompensated disease (14). Exercise (specifically resistance training) may have the added benefit of halting skeletal muscle loss, sarcopenia and frailty which are common in the advanced MAFLD population even though patients are obese (sarcopenic obesity) (13,14). Of note, accelerated skeletal muscle loss and sarcopenia occurs in patients with MAFLD even in the absence of advanced liver disease (15). Finally, finding the willpower and motivation to exercise can be difficult for anyone (not just for patients with MAFLD). Considering depression is present in up to 20% of patients with MAFLD (9), this can further exacerbate the inertia of starting physical activity. This and other guidelines have recognised the value-add of a psychologist or behavioural counsellor to the aforementioned multi-disciplinary team to help achieve sustained long-term behavioural change (6,9,10).
Although Keating et al. provide useful guidance for treating an individual with MAFLD, thought is needed on how these data ought to inform decision making in public health, given that the ability and desire to engage in regular exercise can be influenced by community, corporate, societal, and legislative factors (10). In particular, the required multi-disciplinary approach may not be cost effective, feasible or scalable to tackle MAFLD at a population level. Clearly, there is more work to be done, but position statements such as this one help in the global fight against MAFLD by educating and raising awareness.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-686/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Liu K, McCaughan GW. Epidemiology and Etiologic Associations of Non-alcoholic Fatty Liver Disease and Associated HCC. Adv Exp Med Biol 2018;1061:3-18. [Crossref] [PubMed]
- Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547-55. [Crossref] [PubMed]
- Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367-78.e5; quiz e14-5. [Crossref] [PubMed]
- Keating SE, Sabag A, Hallsworth K, et al. Exercise in the Management of Metabolic-Associated Fatty Liver Disease (MAFLD) in Adults: A Position Statement from Exercise and Sport Science Australia. Sports Med 2023;53:2347-71. [Crossref] [PubMed]
- Hansen D, Coninx K, Beckers P, et al. Appropriate exercise prescription in primary and secondary prevention of cardiovascular disease: why this skill remains to be improved among clinicians and healthcare professionals. A call for action from the EXPERT Network†. Eur J Prev Cardiol 2023;30:1986-95. [Crossref] [PubMed]
- Younossi ZM, Corey KE, Lim JK. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021;160:912-8. [Crossref] [PubMed]
- Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157-66. [Crossref] [PubMed]
- Liu K, Wong VW, Lau K, et al. Prognostic Value of Controlled Attenuation Parameter by Transient Elastography. Am J Gastroenterol 2017;112:1812-23. [Crossref] [PubMed]
- Francque SM, Marchesini G, Kautz A, et al. Non-alcoholic fatty liver disease: A patient guideline. JHEP Rep 2021;3:100322. [Crossref] [PubMed]
- Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023;77:1797-835. [Crossref] [PubMed]
- Lee Y, Doumouras AG, Yu J, et al. Complete Resolution of Nonalcoholic Fatty Liver Disease After Bariatric Surgery: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019;17:1040-1060.e11. [Crossref] [PubMed]
- Bellicha A, Ciangura C, Roda C, et al. Effect of exercise training after bariatric surgery: A 5-year follow-up study of a randomized controlled trial. PLoS One 2022;17:e0271561. [Crossref] [PubMed]
- Laube R, Wang H, Park L, et al. Frailty in advanced liver disease. Liver Int 2018;38:2117-28. [Crossref] [PubMed]
- Duarte-Rojo A, Ruiz-Margáin A, Montaño-Loza AJ, et al. Exercise and physical activity for patients with end-stage liver disease: Improving functional status and sarcopenia while on the transplant waiting list. Liver Transpl 2018;24:122-39. [Crossref] [PubMed]
- Sinn DH, Kang D, Kang M, et al. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: A longitudinal cohort study. Hepatology 2022;76:1746-54. [Crossref] [PubMed]


