
Hepatectomy guided by the diseased bile duct and hepatic vein for hepatolithiasis
Introduction
Hepatolithiasis (HL) is a common and serious disease with high incidence in Southeast Asian countries (1), especially in Southwest China, South China, and Southeast coastal areas of China. Based on the pathological characteristics of the segmented stone distribution and atrophy of the hepatic parenchyma, resection of the atrophied liver involving the diseased bile duct is the most effective and common surgical treatment for HL. As proposed by Shindoh et al., hepatobiliary surgeons have used anatomic liver resection (AR) for hepatocellular carcinoma (HCC), which involves complete resection of the anatomic area supplied by the portal vein of the Glissonean branch (2). However, factors such as changes in bile fluid dynamics, stone compression, and chronic inflammation associated with HL lead to portal vein and hepatic artery stenosis and occlusion in some diseased areas, resulting in the diseased bile duct and hepatic vein (HV) becoming significant characteristic anatomical structures. In such cases, the effect and success rate of indocyanine green fluorescence staining are poor for navigation during HL surgery. Digital imaging techniques such as 3D visualization, 3D printing, and artificial intelligence can facilitate preoperative evaluation but not accurate evaluation of all real anatomical structures and guidance of the transection plane. In such patients with HL, the lack of effective intrahepatic anatomic landmarks used during AR results in residual stones and diseased bile ducts and recurrence of stones. This necessitates repeated surgeries and affects postoperative recovery and patients’ quality of life. Therefore, to allow for standardized and more accurate hepatectomy for HL and provide a theoretical basis for formulating corresponding treatment norms, we proposed and applied hepatectomy guided by the bile duct and HV (H-BV), that is by using the diseased bile duct tree and HV as anatomic landmarks.
Key advantages
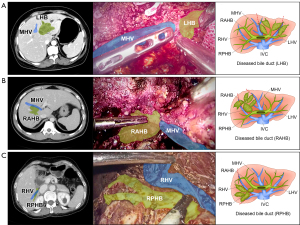
Among a large number of cases in clinical practice, we found that the diseased bile duct was still present in the Glissonean capsule, usually in a phased distributional tree structure, and some portion of the dilated intrahepatic bile duct after liver tissue atrophy was often present close to the major HV, with an obvious anatomical space [bile duct-HV (BV) space] between them (Figure 1A-1C). Therefore, the core of the H-BV philosophy is AR based on the diseased bile duct tree drainage territory. Through detailed evaluation and planning using preoperative 3D visualization technology, one or more liver segments in the region supplied by the diseased bile duct can be jointly excised through intraoperative dissection of the BV space. The key advantage of this approach is that it is appropriate for both classical anatomic resection and the unique pathological and anatomical characteristics of HL; additionally, it provides exact anatomical landmarks for regional HL hepatectomy. It not only ensures complete radical resection of the diseased bile duct of the HL, avoiding intraoperative disorientation and residual stones, but also preserves the functional future liver remnant to the maximum extent and reduces surgical complications.
Specific programs and evidence support
Huang et al. reported that the proportion of dilated bile ducts close to the root or trunk of the middle HV in cases of left intrahepatic bile duct calculus is as high as 40.6%, and that laparoscopic middle HV-guided anatomical hemi-hepatectomy combined with transhepatic duct lithotomy is safe and feasible in such cases (3). Similar results were obtained during our previous studies. HV-guided hepatectomy has certain advantages over traditional laparoscopic hepatectomy for improving intraoperative stone clearance and reducing the recurrence rate of stones and cholangitis (4). H-BV should be performed when the filled stones are distributed in separate regions, the affected liver area is atrophic, and the bile duct is close to the HV. There are specific principles of H-BV that affect clinical treatment. In H-BV, the smallest unit is a subsegment/segment, the largest future liver remnant is preserved, and the drainage area of the diseased bile duct tree is completely removed. The Glissonean capsule is dissected, the boundary between the diseased and normal bile ducts is found to separate the bile duct, artery, and portal vein branches climbing up the diseased bile duct to expose the iconic HV and transect the hepatic parenchyma, and the diseased bile duct and HV are completely separated along the BV space. If the vascular triple structure in the Glissonean pedicle cannot be separately dissected due to inflammation, the diseased bile duct can be incised first to remove the obstructed and compressed stones, and then the arteries and veins can be treated safely.
Barriers
Correct identification of the BV space is the main difficulty and key point. Therefore, extensive hepatectomy experience and a thorough understanding of the pathological and anatomical changes in HL are essential. During the operation, effective treatment should be administered according to specific conditions, and the boundary of the diseased bile duct should be identified to avoid damage to the normal bile duct, which can lead to stenosis. Additionally, the removal of the HV along the BV space can lead to excessive uncontrollable bleeding and the risk of gas embolism during minimally-invasive surgery, especially if inflammation causes the bile duct and HV to become tightly attached. Therefore, the procedure should be performed by a surgeon with sufficient experience with HL surgery and HV exposure technology who can perform vascular suturing and reconstruction; additionally, if uncontrolled bleeding occurs during minimally-invasive surgery, open surgery should be performed.
Conclusions
Herein, we proposed an innovative procedure of hepatectomy guided by the diseased bile duct tree and HV as anatomic landmarks. Multicenter, randomized, controlled clinical trials should be conducted in future to provide high-level evidence to further confirm the advantages of H-BV and improve the short-term outcomes and long-term prognoses of HL.
In the future, the integration of H-BV and technologies such as precision surgery, digital medicine, and accelerated rehabilitation surgery is expected to standardize the surgical theory and technical system of HL and provide standardized and homogenized surgical guidelines for surgeons.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was a standard submission to the journal. The article has undergone external peer review.
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-633/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-633/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kim HJ, Kim JS, Joo MK, et al. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J Gastroenterol 2015;21:13418-31. [Crossref] [PubMed]
- Shindoh J, Makuuchi M, Matsuyama Y, et al. Complete removal of the tumor-bearing portal territory decreases local tumor recurrence and improves disease-specific survival of patients with hepatocellular carcinoma. J Hepatol 2016;64:594-600. [Crossref] [PubMed]
- Huang L, Lai J, Liao C, et al. Classification of left-side hepatolithiasis for laparoscopic middle hepatic vein-guided anatomical hemihepatectomy combined with transhepatic duct lithotomy. Surg Endosc 2023;37:5737-51. [Crossref] [PubMed]
- Liao KX, Chen L, Ma L, et al. Laparoscopic middle-hepatic-vein-guided anatomical hemihepatectomy in the treatment of hepatolithiasis: a 10-year case study. Surg Endosc 2022;36:881-8. [Crossref] [PubMed]


