
Effects of exercise and nutrition in improving sarcopenia in liver cirrhosis patients: a systematic review and meta-analysis
Highlight box
Key findings
• Exercise and nutrition interventions significantly enhance sarcopenia outcomes in liver cirrhosis patients, notably improving skeletal muscle index and albumin levels, particularly with treatments extending beyond 12 months.
What is known and what is new?
• Sarcopenia is highly prevalent among liver cirrhosis patients and significantly affects their quality of life and life expectancy, with inconsistencies reported in the effectiveness of common interventions such as exercise and nutrition.
• This manuscript highlights that long-term exercise and nutrition interventions (12 months or longer) are particularly effective in improving skeletal muscle index and albumin levels, thus significantly enhancing the management of sarcopenia in liver cirrhosis patients.
What is the implication, and what should change now?
• These insights advocate for the integration of exercise and nutritional strategies into the routine management of liver cirrhosis to combat sarcopenia effectively. Healthcare professionals should adapt these findings into practice, promoting personalized, long-term intervention plans. Future studies should aim to optimize these interventions for individual patient profiles, enhancing therapeutic outcomes.
Introduction
Liver cirrhosis (LC) is a worsening health problem that poses a life-threatening risk to individuals (1). According to the World Health Organization (WHO) report in 2022, LC leads to 1.34 million deaths annually, although these figures may be underestimated (2). Recent reports have highlighted the significance of sarcopenia among LC patients (3,4). Sarcopenia, characterized by muscle wasting, encompasses the decline in muscle mass, strength, and physical performance (5,6). In LC, the incidence of sarcopenia ranges from 48% to 70% (7,8), and skeletal muscle wasting is more pronounced in these patients compared to the general elderly population due to impaired liver function (3). LC with sarcopenia results from various factors, including malnutrition, malabsorption, alterations in lipid and peptide metabolism, reduced clearance of hepatamine, increased cytokine levels, elevated myostatin secretion, decreased metabolic hormone secretion, and insufficient exercise (9,10). This condition is associated with increased mortality rates, higher hospitalization rates, poorer prognosis following liver transplant, reduced quality of life, and an elevated risk of developing other complications (9,11). Moreover, LC with sarcopenia contributes to the higher mortality rates observed in Asian populations compared to Western populations (8). Various approaches were employed to reduce sarcopenia, including exercise, nutrition, testosterone replacement, and transjugular intrahepatic portosystemic shunt (TIPS) (12). However, due to the expensive nature of invasive testosterone replacement and TIPS procedures, exercise and nutrition appear to be more cost-effective, non-invasive, and sustainable alternatives. Engaging in physical activity can promote the release of insulin-like growth factor 1 (IGF-1), which has an anabolic effect and enhances muscle strength and functionality (9,13). Liver function plays a role in energy metabolism changes, leading to the breakdown of amino acids and protein deficiency (14). Nonetheless, the effectiveness of exercise or nutrition in reducing LC in individuals with sarcopenia has yet to demonstrate consistent results (15-17).
Sarcopenia is a well-recognized condition associated with cancer, diabetes, chronic obstructive pulmonary disease, and chronic renal disease (18). However, there has been a growing recognition of sarcopenia as an important issue among individuals with chronic liver disease in recent years (19). Previous studies have indicated that sarcopenia and pre-sarcopenia, characterized by the loss of muscle volume and strength, are not uncommon in patients with chronic liver disease, even in the early stages of the disease (19). The pathogenesis of sarcopenia in patients with LC can be attributed to three main factors: imbalanced intake, metabolic disorders, and malabsorption. Imbalanced intake may manifest as symptoms such as nausea, early satiety, and loss of appetite, which could be influenced by the presence of ascites, elevated levels of leptin, and tumor necrosis factor-alpha (TNF-α) (20). Leptin and TNF-α have been associated with appetite loss (21), while altered taste perception has been linked to zinc deficiency (22). Additionally, dietary salt restriction, reduced protein intake, and iatrogenic fasting can have an impact on dietary intake (21). Metabolic disorders refer to increased metabolic demands, where a fasting state may lead to higher rates of fat oxidation and amino acid gluconeogenesis due to depleted hepatic glycogen storage. Moreover, the presence of ascites and portal hypertension may cause more than 12% of patients to experience a static energy expenditure increase exceeding the expected value in 34% of cases. Malabsorption is primarily attributed to slower excretion of bile salts (21).
The aim of this study was to systematically review and conduct a meta-analysis of existing evidence to determine the effectiveness of exercise and nutrition in reducing sarcopenia among patients with LC. We present this article in accordance with the PRISMA reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-639/rc).
Methods
Protocol and registration
The systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) with registration number of CRD42022330747.
Eligibility criteria
All the included articles must discuss the effect of an exercise intervention on patients with LC complicated with sarcopenia. The eligibility criteria were followed the Population, Intervention, Controls, Outcome (PICO) characteristics.
The inclusion criteria were: (I) patients with LC complicated with sarcopenia; (II) the type of studies was only randomized controlled trial (RCT); (III) the intervention was exercise or nutrition; (IV) evaluation measurements include objective outcomes, such as biological indices and physical ability (muscle strength, muscle size, and walking test) and/or liver function indicators [e.g., albumin, glutamic oxaloacetic transaminase (GOT), and glutamic pyruvic transaminase (GPT)]. All articles were available until December 2021 that met the inclusion criteria and no language limitation.
The exclusion criteria were: (I) the population was not adults; (II) the interventions were combined with others and not to clarify the exercise or nutrition effect.
Resources and search strategy
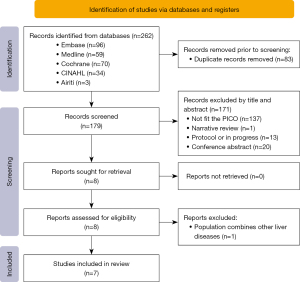
The following five databases were searched for studies: Embase, Medline OVID, Cochrane & Central, CINAHL, and Airiti Library (Traditional Chinese). The primary keywords were liver cirrhosis, sarcopenia, exercise, and nutrition. The controlled vocabularies from Emtree, MeSH, and CINAHL Subject Heading were also assembled to generate more relevant searches. The search process was shown in Figure 1. To consider the new studies published after December 2021, the researchers also rechecked the publications related to the topic till 15th May 2022.
Study selection
The articles were screened using EndNote software X version and one of authors (Y.L.C.) removed the duplications after pooling all the outputs of the resources. Two authors (Y.L.C. and H.C.H.) reviewed the title and abstract, full text articles independently according to the inclusion and exclusion criteria and compared the difference of results. The final included articles confirmation by discussion after the third author (H.F.L.) joined and achieved consensus. A total of 262 studies were electronically found while 83 duplications were removed by EndNote software. Then, 140 studies were excluded because the unsuitable title and abstract. Thirty-one full texts were assessed for eligibility then finally seven RCT were included in the analyzed. The reasons of excluded full texts were such as general reviews, protocol or in progress, insufficient data for meat-analysis, and interventions combined other non-exercise or nutrition.
Data extraction and appraisal for study quality
One author (Y.L.C.) extracted the information of the studies, and another author (L.H.C.) rechecked the correct of the extracted results. The extracted content was such as first authors̓ name, publication year, country, research design, age with participants, strategies duration, case numbers, interventions, finding of critical variables for each article (Table 1). The data for meta-analysis were also extracted and re-calculated and transformed into mean and standard deviation if the original data were median, range, or percentile 25–75. The change-from-baseline standard deviation were calculated by the formular suggested by Cochrane.
Table 1
| Studya | Identification of studyb | Interventionc | Main finding |
|---|---|---|---|
| Zenith et al., 2014/Canada/1.c | 1. Randomized controlled trial; 2. 58 with liver cirrhosis (C-P A or B); 3. 8 weeks; 4. E=9; C=10 | Experimental group: Monark cycle ergometer training 3 days per week Control group: no intervention Exercise: Monark cycle ergometer at a power output equal to the heart rate at 60–80% of baseline peak VO2. After a 5-minute warm-up of low-level cycling, exercise was initiated at 30 minutes per session and increased by 2.5 minutes per session each week until study completion |
(I) Variables of sarcopenia: (i) between groups: significant: thigh circumference (95% CI: 0.54 to 1.56); not significant: 6MWD (95% CI: −12.4 to 59.4), BMI (95% CI: −0.7 to 0.6) between E and C groups. (ii) For E group: the improved significantly variables were 6MWD, thigh circumference (II) Variables of liver function: there was no significance for albumin (95% CI: −1.4 to 2.4), GPT (95% CI: −9.8 to 8.0), and GOT (95% CI: −6.1 to 16.2) between and within groups (III) Others: all cardiorespiratory function were improved significantly (P=0.001) between E and C but peak heart rate and peak systolic blood pressure |
| Aamann et al., 2019/Denmark/1.c | 1. Randomized controlled trial; 2. 62 with liver cirrhosis (C-P A or B); 3. 12 weeks; 4. E=20; C=19 | Experimental group: resistance training 3 days per week + nutritional supplements + registration of daily activity Control group: nutritional supplements + registration of daily activity Exercise: the muscle-strengthening activity was performed on 3 nonconsecutive days per week during a 12-week period Diet: oral nutritional supplements (nutri drink compact protein 125 mL; Nutricia A/S, Alleroed, Denmark) containing 14.4 g protein (2.9-g branched-chain amino acids) per 100 g were added |
(I) Variables of sarcopenia: (i) between groups: significant: no; not significant: 6MWD (95% CI: −15.6 to 53.2), BMI (95% CI: −0.6 to 1.1), midarm circumference (95% CI: −0.9 to 1.0), thigh circumference (95% CI: −0.4 to 2.2), and calf circumference (95% CI: −0.6 to 0.9). (ii) For E group: the significantly improved variables were 6MWD, BMI, midarm circumference, thigh circumference, and calf circumference (II) Variables of liver function: there was no significance for albumin, GOT, and GPT between and within E and C group (III) Others: knee extension peak torque was improved in E than C (95% CI: 0–22) |
| Chen et al., 2020/America/1.c | 1. Randomized controlled trial; 2. 56 with liver cirrhosis (C-P B or C); 3. 12 weeks; 4. E=9; C=8 | Experimental group: counseling physical training (home-based physical activity program) + nutritional supplements Control group: nutritional supplements Exercise: CPET was used to assess aerobic fitness Diet: total protein intake of 1.2–1.5 g/kg/day, a light snack at night, and a drink supplement providing 6 g of essential amino acids twice a day (total of 12 g/day) for the 12-week intervention period |
(I) Variables of sarcopenia: (i) between groups: significant: 6MWD (95% CI: 98.56 to 201.44); not significant: SMI (95% CI: −8 to 12) and BMI (95% CI: −2.9 to 3.1). (ii) For E group: all variables were not significant (II) Variables of liver function: no measurement (III) Others: there was no significance for total sickness impact profile score between and within groups |
| Macias-Rodriguez et al., 2020/Mexico/1.c | 1. Randomized controlled trial; 2. 54 with liver cirrhosis (C-P A) and portal hypertension; 3. 8 weeks; 4. E=22; C=21 | Experimental group: non-alcoholic beer (330 mL/per day) + dietary plan + exercise program Control group: water (330 mL/per day) + dietary plan + exercise program Exercise: aiming to reach >2,500 steps/d above the average baseline level and a total number of steps of 5,000/d. Intensity of exercise was set using the Borg Scale of perceived exertion (scale from 6 to 20), and each patient was trained to reach a target intensity of 10–12 (corresponding to light to moderate intensity and from 3 to 3–6 METs Diet: 60% of carbohydrates, 1.3–1.5 g of protein/kg body weight/d, and the rest from lipids |
(I) Variables of sarcopenia: (i) between groups: significant: no variable; not significant: thigh circumference (95% CI: −.85 to 4.05), handgrip (95% CI: −3.42 to 4.82), midarm circumference (95% CI: −1.04 to 1.64), sit-to-stand (95% CI: −1.45 to 3.45). (ii) For E group: the significantly improved variables were thigh circumference, midarm circumference, handgrip, sit-to-stand (II) Variables of liver function: there was significant change for albumin but not significant for GOT and GPT between groups. However, there was no significant change for albumin and GPT but significant for GOT in E group (III) Others: the improvement in quality of life was higher in the E group |
| Hernandez-Conde et al., 2021/Spain/1.c | 1. Randomized controlled trial; 2. 65 with liver cirrhosis (C-P A); 3. 12 weeks; 4. E=15; C=17 | Experimental group: BCAA supplements + physical activity Control group: placebo supplements + physical activity Exercise: 5,000–10,000 steps/day, with gradual increments of 2,000–2,500 steps/day, moderate intensity exercise in 30-minute sessions to achieve at least 150 min/w Diet: in experimental group, 15 g of protein, 8.5 g of fat, 68 g of carbohydrates, BCAA (2.61 g of leucine, 1.01 g of isoleucine, and 1.62 g of valine), and additional vitamins and minerals (including zinc). In control group, maltodextrin 99.63%, beta-carotene colorant 1% 0.05%, aspartame sweetener 0.07%, and vanilla aroma 0.25% |
(I) Variables of sarcopenia: (i) between groups: significant: SMI (P=0.022); not significant: BMI (P=0.158). (ii) For E group: the significantly improved variables were BMI (P<0.001) and SMI (P=0.023) (II) Variables of liver function: there was significant change for albumin in E group (P=0.007) but not significant between E and C groups (P=0.091) (III) Others: the improvement in BMI was significant between E and C group (ΔSMI: 2.3 vs. 0.1, P=0.022) as well as in E group (P<0.01) |
| Lattanzi et al., 2021/Italy/1.c | 1. Randomized controlled trial; 2. 58 with liver cirrhosis (C-P A) and portal hypertension; 3. 12 weeks; 4. E=14; C=10 | Experimental group: HMB supplements 3 g/day Control group: placebo supplements (sorbitol 3 g/day) |
(I) Variables of sarcopenia: (i) between groups: no comparison; (ii) for E group: the significantly improved variables were thickness pressure index (P<0.05), 6WMD (P<0.05) but not significant for BMI, lean mass, hand grip, and five-chair stand test (II) Variables of liver function: no measurement (III) Others: there was significant change for liver frailty index in E group (P<0.05) |
| Okubo et al., 2021/Japan/1.c | 1. Randomized controlled trial; 2. 72 with decompensated cirrhosis and oral BCAA treatment at least 6 months and would be continued; 3. 12 months; 4. E=15; C=17 | Experimental group: oral vitamin D supplements (vitamin D supplementation 2,000 IU/day) Control group: no intervention |
(I) Variables of sarcopenia: (i) between groups: significant: SMI (P=0.002) and handgrip (P=0.009). (ii) For E group: prevalence rate of sarcopenia was decreased significantly (P=0.002) (II) Variables of liver function: there was no significant change for albumin in E group (P=0.712) (III) Others: there was significant change for serum vitamin D in E group (P=0.0009) |
a, author, year/country/level of evidence; b, 1. design; 2. age (years) with participants; 3. duration; 4. case number; c, experimental group; control group. C-P, Child-Pugh; E, experimental group; C, control group; CI, confidence interval; 6MWD, six-minute walk test; BMI, body mass index; GPT, glutamic pyruvic transaminase; GOT, glutamic oxaloacetic transaminase; CPET, cardiopulmonary exercise testing; MET, metabolic equivalent; BCAA, branched-chain amino acid; HMB, β-hydroxy-β-methyl butyric acid; SMI, skeletal muscle index.
The quality of included studies was assessed by the Cochrane risk of bias (ROB) 2.0 version to determine ROB for each study in order to classify them into ROB is low, some concerns, or high. A total of seven items for appraisal the quality which were: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
Statistical analysis
The qualitative information was synthesized according to the characteristics of participants, strategies, and effectiveness as well as meta-analysis for quantitative information.
The meta-analysis was performed using the Cochrane Collaboration software (Review Manager 5.4 version for Windows), which used the inverse variance method. Mean difference (MD) and standard deviation were used as the statistical effect quantity, and 95% confidence intervals (CIs) were calculated by effect analysis. The statistical heterogeneity was calculated and presented as I2, which is an approximate measure of the proportion of the variation due to heterogeneity rather than sampling error. When I2 more than 50%, it was considered substantial heterogeneity (23).
Results
A total of 262 studies were searched from five electronic databases and seven articles were included for data extraction finally (Figure 1). All interventional studies were RCT. Therefore, the sample size of eligible studies for meta-analysis ranged from 17 to 43; totally, 206 subjects were meta-analyzed (104 cases and 102 controls). The age range of the participants was 54 to 72 years old. The interventions were exercise for 1 article, nutrition for 2 articles, and combined exercise and nutrition for 4 articles. The publication bias was low risk for 5 studies and some concern for 2 ones using ROB 2.0 appraisal (Figure 2).
The eligible papers were evaluated in two main sections: variables of sarcopenia and liver functions. Variables of sarcopenia included skeletal muscle index (SMI), hand grip, the six-minute walk test (6MWD), sit-to-stand, thigh/midarm/calf circumference, body mass index (BMI). Liver functions included liver biochemical testing value, like albumin and GOT/GPT.
The characteristics of included studies related to the exercise and nutrition for LC with sarcopenia was shown in Table 1. The effectiveness of exercise and nutrition strategies for LC with sarcopenia between experimental and control group as well as in experimental group was present in Table 2.
Table 2
| Variable | Zenith et al., 2014; I: E; C: no | Aamann et al., 2019; I: E+N; C: E+N | Chen et al., 2020; I: E+N; C: N | Macías-Rodríguez et al., 2020; I: E+N; C: E+N | Hernández-Conde et al., 2021; I: E+N; C: E | Lattanzi et al., 2021a; I: N; C: no | Okubo et al., 2021; I: N; C: no |
|---|---|---|---|---|---|---|---|
| Between intervention and control groups | |||||||
| Sarcopenia | |||||||
| SMI | − | + | + | ||||
| 6WMD | − | − | + | ||||
| Handgrip | − | + | |||||
| BMI | − | − | − | − | |||
| Thigh circumference | + | − | − | ||||
| Midarm circumference | − | − | |||||
| Sit-to-stand | − | ||||||
| Calf circumference | − | ||||||
| Liver function | |||||||
| Albumin | − | − | |||||
| GOT | − | ||||||
| GPT | − | ||||||
| In intervention group | |||||||
| Sarcopenia | |||||||
| SMI | − | + | + | ||||
| 6WMD | + | + | − | + | |||
| Handgrip | + | − | |||||
| BMI | + | − | + | − | |||
| Thigh circumference | + | + | + | ||||
| Midarm circumference | + | + | |||||
| Sit-to-stand | + | ||||||
| Calf circumference | + | − | |||||
| Liver function | |||||||
| Albumin | − | − | − | + | − | ||
| GOT | − | − | + | ||||
| GPT | − | − | − | ||||
a, the comparison was only within group rather than between groups. +, effectiveness; −, non-effectiveness. I, intervention group; C, control group; E, exercise; N, nutrition; SMI, skeletal muscle index; 6MWD, six-minute walk test; BMI, body mass index; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase.
Strategies of exercise and nutrition
Exercise
A total of five studies were provided exercise strategies (15,17,24-26). The purpose of exercise program was muscle-strengthening, increasing cardiopulmonary function and walking steps. The courses were 8–12 weeks and duration time ranged 30 to 50 minutes.
Nutrition
A total of six studies were provided nutrition strategies (15,24-28). The ingredients were such as protein, branched-chain amino acids (BCAAs), carbohydrates, vitamins, β-hydroxy β-methylbutyric acid. The courses were 8–12 weeks; however, the quantity was inconsistent.
Effectiveness of decreasing sarcopenia
Some indicators were used to measure the effectiveness of sarcopenia which are SMI, hand grip, 6MWD, sit-to-stand, thigh/midarm/calf circumference, and BMI. SMI is the major indicator of sarcopenia, followed by hand grip, 6MWD, sit-to-stand. There were three articles for SMI (24,26,28); three for hand grip (26-28); four for 6MWD (15,17,24,27); two for sit-to-stand (26,27); three for thigh circumference (15,17,26); two for midarm circumference (15,26); two for calf circumference (15,27); five for BMI (15,17,24,25,27). The effectiveness of decreasing sarcopenia for various indicators were shown in Figure 3.
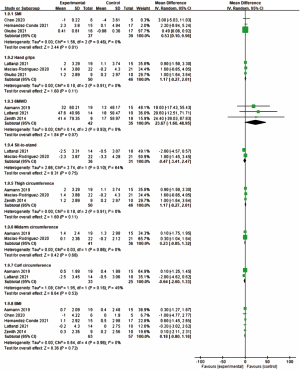
SMI
Three studies were included in the analysis (24,25,28). For the interventions, two studies were using exercise combined nutrition (24,25) and one is only nutritional support (28). The result of meta-analysis was shown it was significant between experiment and control (MD: 0.53, P=0.01, 95% CI: 0.10, 0.96, I2=0%). However, the duration of the strategies were 12 weeks for two studies (24,25) but one is 12 months (28), the sensitivity analysis was examined after removing Okubo et al. [2021] and the results were shown the effect of decreasing sarcopenia was not significant (28). For the experiment group, two of three studies showed the positive effects with nutrition interventions (25,28) but one did not with exercise (24).
Hand grip
Three studies were included in the analysis (26-28). For the interventions, one study was using exercise combined nutrition (26), two were only nutritional support (27,28). The result of meta-analysis was shown it was not significant between experiment and control (MD: 1.17, P=0.11, 95% CI: −0.27, 2.61, I2=0%).
6MWD
Four studies measured this outcome (15,17,24,27), however, only three studies were included in the analysis (15,17,27), because the mean and the standard deviation which presented by Chen et al. (24) were different from other studies. If we included four studies to conduct data analysis, the heterogeneity would increase to 84%. For the interventions, one study was using exercise combined nutrition (15), another was only nutritional support (27), and the other was only exercise strategy (17). The result of meta-analysis was shown it was significant between experiment and control (MD: 55.08, P=0.07, 95% CI: −3.65, 113.81, I2=84%).
For the experiment group, three (15,17,27) of four studies showed the positive effects but one (24) did not. However, the interventions were not specific.
Sit-to-stand
Two studies were included in the analysis (26,27). For the interventions, one study was using exercise combined nutrition (26), and the other is only nutritional support (27). The result of meta-analysis was shown it was not significant between experiment and control (MD: −0.47, P=0.75, 95% CI: −3.41, 2.47, I2=64%). Only one study (15) measured the indicator and showed the positive effect, the time was decreased after intervention.
Thigh circumference
Three studies were included in the analysis (15,17,26). For the interventions, two studies were using exercise combined nutrition (15,26) and one was only exercise strategy (17). The result of meta-analysis was shown it was not significant between experiment and control (MD: 1.17, P=0.11, 95% CI: −0.27, 2.61, I2=0%). For the experiment group, all three studies (15,17,26) with exercise or combined nutrition showed that the positive results, and the circumference was increased after intervention.
Midarm circumference
Two studies were included in the analysis (15,26). For the interventions, two studies were using exercise combined nutrition (15,26). The result of meta-analysis was shown it was not significant between experiment and control (MD: 0.23, P=0.68, 95% CI: −0.85, 1.32, I2=0%). For the experiment group, two studies (15,26) with combined exercise and nutrition interventions showed that the positive results, the circumference was increased after intervention.
Calf circumference
Two studies were included in the analysis (15,27). For the interventions, one study was using exercise combined nutrition (15), and the other was only nutritional support (27). The result of meta-analysis was shown it was not significant between experiment and control (MD: −0.64, P=0.53, 95% CI: −2.60, 1.33, I2=49%). For the experiment group, two studies showed that the inconsistent results (26,27).
BMI
Five studies were included in the analysis (15,17,24,25,27). For the interventions, three studies were using exercise combined nutrition (15,24,25), another was only nutritional support (27), and the other was exercise strategy (17). The result of meta-analysis was shown it was significant between experiment and control (MD: 0.18, P=0.72, 95% CI: −0.80, 1.16, I2=0%). For the experiment group, two (15,25) of four studies showed the positive effects with nutrition interventions but two (24,27) did not with exercise or nutrition.
Effectiveness of liver function
Some indicators were used to measure the liver function, including albumin, GOT, GPT. There were four articles for albumin (15,17,25,26); three for GOT and GPT (15,17,26). The effectiveness of improving liver function for various indicators were compared in Figure 4.
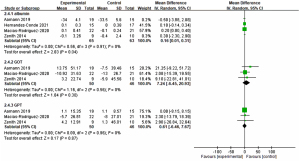
Albumin
Four studies were included in the analysis (15,17,25,26). For the interventions, three studies were using exercise combined nutrition (15,25,26) and one was only exercise strategy (17). The result of meta-analysis was shown it was significant between experiment and control (MD: 0.16, P=0.04, 95% CI: 0.01, 0.31, I2=0%). For the experiment group, only one (25) of five studies showed the positive effect, indicating albumin could be improved after providing intervention.
GOT
Three studies were included in the analysis (15,17,26). For the interventions, two studies were using exercise combined nutrition (15,26) and one was only exercise strategy (17). The result of meta-analysis was shown it was significant between experiment and control (MD: 7.24, P=0.30, 95% CI: −6.45, 20.93, I2=0%). In the experimental group, only one of three studies showed that GOT values was decreased after the intervention.
GPT
Three studies were included in the analysis (15,17,26). For the interventions, two studies were using exercise combined nutrition (15,26) and one was only exercise strategy (17). The result of meta-analysis was shown it was significant between experiment and control (MD: 0.61, P=0.87, 95% CI: −6.46, 7.67, I2=0%). In the experimental group, all three studies showed that GOT values was not decreased after the intervention.
Discussion
In this systemic review, we found that, regardless of whether nutrition and exercise were combined or only a single nutrition or exercise strategy was involved, only SMI and albumin were significantly higher in the intervention group compared to control group. For difference between pre- and post-intervention among the experimental group, certain indicators that can be effectively improved under intervention are thigh circumference, midarm circumference, and sit-to-stand. The current mechanism for sarcopenia in patients with cirrhosis is muscle exhaustion caused by altered protein turnover, energy disposition, and hormonal and metabolic changes (10,11). Thus, exercise and nutrition strategies can indeed help reduce sarcopenia in patients with cirrhosis. However, the control group also had interventions in nutrition or exercise in four articles, so that there may be a smaller difference between the two groups. This also implies that nutrition and exercise may be helpful in reducing sarcopenia.
Both sarcopenia working groups, which are European Working Group on Sarcopenia in Older People (EWGSOP) and Asian Working Group for Sarcopenia (AWGS) have been listed the diagnostic criteria of sarcopenia in 2010, it is mainly based on the individual’s quality of skeletal muscle (SMI), muscle performance (hand grip), and walking speed (6WMD). SMI is the main indicator of sarcopenia. Among the meta-analysis, three articles measured SMI; two of which (25,28) found significant difference in comparing the two groups and also significant improvement in the experimental group between pre- and post-intervention. The other one among the three, Chen et al. [2020] showed that there was no significant pre- and post-test comparison between the two groups or the experimental group. This insignificant finding could be due to the characteristics of study population. Most articles included cirrhosis patients with Child-Pugh A or B, except Chen et al. [2020] with Child-Pugh B/C, and Okubo et al. [2021] not explained. The severity of LC is often classified by the Child-Pugh score, which consisted of hepatic encephalopathy, ascites, total bilirubin, albumin and coagulation function (29). Therefore, in addition to improving sarcopenia through nutrition or exercise intervention, it was also found that albumin was significantly different after intervention, which indirectly proves that intervention strategies also have an effect on liver function to improve to a certain extent or slow down the rate of deterioration. Due to the high severity of Child-Pugh C, the inference also makes it difficult to detect differences in efficacy, which requires further verification in future studies. Frailty is another condition often resulting from sarcopenia. The Liver Frailty Index (LFI) serves as a straightforward and manageable indicator of vulnerability, incorporating assessments of lower limb strength and static balance. The LFI is based on three performance-oriented tests: grip strength, chair stand, and balance tests (30), and it classifies physical function into robust, pre-frail, or frail categories using specified LFI cut-off values (31). Studies have shown that lower limb strength and static balance are more pertinent than grip strength when evaluating mobility outcomes (32). This supports our observation that interventions can effectively improve thigh circumference, midarm circumference, and sit-to-stand performance—three key indicators. Furthermore, the LFI has been identified as a useful tool for predicting mortality in patients with end-stage liver disease (33) and has also been validated by Hirota et al. as effective for screening muscle atrophy in chronic liver disease patients with hepatocellular carcinoma (HCC) (34).
Exercise can increase local skeletal muscle blood flow, produce more glucose, and stimulate the expression of skeletal muscle glucose transporter GUL4, so that more glucose can enter skeletal muscle for storage in the form of glycogen (35). Myostatin can inhibit the biosynthesis of skeletal muscle protein, and its concentration in the body is negatively correlated with the amount of human muscle. Therefore, exercise can inhibit the mRNA production of myostatin. When resistance training reaches 60–85%, it can inhibit the performance of myostatin (36). Acute resistance exercise will stimulate protein synthesis, the proliferation and differentiation of satellite cells in skeletal muscle, in order to promote muscle hypertrophy, thickness and strength performance (37). Resistance exercise seems to be one of measure tool of sarcopenia. Soldera et al. found that comparing resistance exercise group, the normal activity group, and the control group, there was a significant difference in the increase in muscle strength and muscle thickness between the resistance exercise group and the control group, but no significant difference in muscle mass (muscle strength per unit muscle) (38). Aamanne et al. also showed that resistance training (3 times/weeks for 12 weeks) can increase 6MWD significantly. Therefore, exercise can increase muscle growth and muscle strength (39). Additionally, exercise programs can be tailored as personalized courses within hospitals, complemented by other treatments for patients with liver disease. Narao et al. have shown that in-hospital physical therapy tailored to personal intensity levels can enhance muscle strength, as evidenced by improvements in bilateral knee extension strength, chair stand test performance, and functional independence measure (FIM) scores, all without exacerbating liver function in patients with HCC (40). Such in-hospital exercises have also been noted to ameliorate frailty in patients with HCC (41). Moreover, when combined with BCAA treatment, in-hospital exercise can effectively reduce skeletal muscle atrophy during HCC treatment in patients with chronic liver disease. Cancer rehabilitation, an innovative multidisciplinary approach involving nutritional and physical therapy, has been proven to extend survival in patients with HCC (42). Additionally, Koya et al. demonstrated that in-hospital exercise could increase muscle mass and prevent sarcopenia in HCC patients undergoing transcatheter arterial chemoembolization (43). In Koya et al.’s study, patients at risk of HCC rupture, those with a history of hepatic encephalopathy, and those graded 2–4 according to the West Haven Criteria were excluded (44). From this, it can be inferred that the limited effectiveness of exercise may be linked to a high Child-Pugh score, as suggested by our study’s findings. Nonetheless, exercise remains beneficial for patients with liver dysfunction, helping to slow the progression of muscle atrophy.
Regarding the mechanism of liver function, both exercise and nutritional intervention should be able to improve sarcopenia. However, the meta-analysis results show that although it cannot be confirmed that exercise combined with nutrition strategy has the greatest effect, either intervention has no effect. For LC patients, though diet and exercise are important strategies for improving sarcopenia, exercise may increase blood ammonia concentration and hepatic portal venous pressure, which may have negative effects (45). Therefore, under the current evidence, the most effective strategy for improving sarcopenia is to combine exercise and nutritional supplementation, and to provide amino acid or protein supplementation after exercise can increase the amount of protein synthesis in the whole body. For exercise options, regardless of increase daily number of steps, resistance training, combined with stretching, strength training, balance training and endurance training, above the options can improve sarcopenia. Meanwhile, the intervention time should be more than 12 months in order to truly reduce sarcopenia in LC patients.
Nutrition supplement can be chosen to improve sarcopenia and liver function. Most nutrition supplying strategies are designed to ensure daily protein intake or nutritional supplements. However, high protein diet may increase the ammonia level and then induce hepatic encephalopathy. The decreased serum ratio of BCAAs to aromatic amino acids (AAAs) has long been considered a key indicator of LC. This imbalance is attributed to various factors, including diminished nutritional intake, hypermetabolism, and the detoxification of ammonia in skeletal muscle (46). A low serum BCAA/AAA ratio impairs the biosynthesis and secretion of albumin in hepatocytes and is also linked to the prognosis of patients with chronic liver disease (47). Kawaguchi et al. have demonstrated that BCAA supplementation not only enhances the nutritional status but also improves the prognosis and quality of life for patients with LC (48). Thus, an imbalance of amino acids poses a significant risk factor for the development of HCC in individuals with cirrhosis. Supplementation with BCAAs has been shown to reduce the risk of HCC and extend the survival of patients with cirrhosis (49). Common supplements include BCAA, β-hydroxy-β-methyl Butyric acid (HMB), etc. Meta-analysis by Chen et al. (24) suggested late evening diet with calories 210 kcal and 12 g BCAA in LC patients. Okubo et al. (28) found that long-term BCAA for 1 year and vitamin D for half year can bring more significant results. Vitamin D can improve skeletal muscle volume and strength in patients with decompensated cirrhosis (50). Unfortunately, BCAA compliance was limited by availability, cost, and gastrointestinal symptoms such as bloating, nausea, and abdominal pain. Macías-Rodríguez et al. (26) takes advantage of non-alcoholic beer for improving nutritional status and endothelial function associated with general nutrition and exercise support among LC patients. Non-alcoholic beer has several nutrients, including vitamin B, minerals and flavonoids, rendering it an attractive nutritional supplement in patients with cirrhosis (51). Therefore, non-alcoholic beer provides better hydration and higher content of minerals, improving muscle function and therefore facilitating exercise performance. Besides, some micronutrients, such as vitamin B12, are considered essential for muscle formation. They can play a role as an antioxidant, and improve exercise tolerance, and neuromuscular function (26).
Metabolic dysfunction is a key mechanism underlying sarcopenia. Metabolic dysfunction-associated steatotic liver disease (MASLD) is the latest term for steatotic liver disease associated with metabolic syndrome. MASLD, previously known as non-alcoholic fatty liver disease (NAFLD) and more recently as metabolic associated fatty liver disease (MAFLD), are both conditions closely linked to metabolic dysregulation (52). It stands as a significant contributor to cirrhosis, positioning itself as the predominant cause of chronic liver disease and a leading factor for liver-related morbidity and mortality, as well as for esophageal squamous cell carcinoma (53). Diagnosis of MAFLD hinges on the identification of fatty liver in conjunction with one of the following metabolic dysfunctions: overweight/obesity, the presence of type 2 diabetes mellitus, or lean/normal weight with evidence of metabolic dysregulation, as outlined in an international expert consensus statement (54,55). Besides, metabolic dysfunction also influences on worsening atherosclerotic cardiovascular disease and sustained virologic response in patients with hepatitis virus (56,57). The relationship between sarcopenia and MASLD is notably bidirectional, sharing common pathophysiological threads, notably insulin resistance, physical inactivity, and obesity (58,59). Sarcopenic obesity emerges as an independent risk factor for MASLD, while the coexistence of sarcopenia with visceral fat obesity and myosteatosis significantly correlates with MASLD, even in non-obese individuals (60). Conversely, a longitudinal study highlighted that patients with MASLD are at an elevated risk for sarcopenia, exhibiting accelerated muscle mass loss over five years compared to those without MASLD (61). Moreover, sarcopenia prevalence was higher in patients with metabolic-associated steatohepatitis (MASH) than in those with MASLD without MASH and control subjects (62). Additionally, the presence of sarcopenia in MASLD patients was strongly associated with significant and advanced liver fibrosis (62,63), underscoring sarcopenia as a potent predictor of mortality and morbidity in MASLD, MASH, and cirrhosis cases (64,65). The primary goal in treating MASLD is lifestyle modification, aiming for a weight reduction of 7% to 10%, especially in individuals with overweight/obesity. Such a significant weight loss has been linked to the resolution of Metabolic Associated Steatohepatitis (MASH) and histological improvement (66). Conversely, a notable characteristic of sarcopenia is skeletal muscle loss. Weight loss strategies that involve energy restriction without concurrent exercise may exacerbate muscle loss, highlighting a critical consideration in treatment approaches. The recommended treatment for sarcopenia includes resistance exercise coupled with optimized nutrition intake (67). Engaging in increased physical activity and maintaining a healthy diet are strategies associated with a lowered risk of both sarcopenia and MASLD, potentially reducing the risk of significant liver fibrosis (63,68). Adequate protein intake is advised for patients with sarcopenia, however, protein supplementation, in the absence of resistance exercise, does not effectively improve muscle mass and strength (69). In contrast, BCAA supplementation offers particular benefits for individuals with hepatic encephalopathy and advanced cirrhosis (Bischoff et al., 2020). The European Society for Clinical Nutrition and Metabolism endorses the Mediterranean diet for managing MASH, citing its benefits in improving insulin resistance and steatosis (70). Furthermore, the Mediterranean diet is also recognized for its positive impact on muscle mass and function in individuals with sarcopenia (71), suggesting a synergistic approach in dietary recommendations for addressing both conditions.
This study, aimed at alleviating or delaying the progression of sarcopenia in patients with cirrhosis, is subject to several limitations that warrant careful consideration. The primary challenge in sarcopenia research, compounded by the scarcity of suitable animal models and the lack of a universally accepted definition and consistent diagnostic criteria, significantly restricts our ability to comprehensively investigate this disorder and develop targeted interventions. This gap hinders clinicians’ ability to establish effective therapeutic strategies or implement preventive measures (72). Indeed, the limited number of articles included in our analysis underscores the need for more interventional studies to robustly ascertain the efficacy of interventions in the future. Additionally, the studies considered had relatively small participant populations, and the intervention durations, ranging from 8–12 weeks, were not sufficiently long to observe the long-term effectiveness of treatments. Furthermore, a significant limitation highlighted by our meta-analysis is the high heterogeneity among the included studies, stemming from the varied protocols of nutrition interventions—ranging from BCAA supplements to non-alcoholic beer—and diverse exercise interventions. This heterogeneity introduces challenges in drawing strong conclusions. For these reasons, the most definitive message we can harvest from this meta-analysis is that sarcopenia may be effectively lessened by evaluating SMI and albumin levels, regardless of the combination of nutrition and exercise interventions. Future research should aim to employ a repetitive modality approach to confirm significant improvements and extend the duration of interventions. This is crucial not only to assess treatment efficacy thoroughly but also to prevent secondary sarcopenia from cirrhosis, thereby enhancing patient outcomes and guiding future research.
Sarcopenia, a condition shared by various diseases that we tempt to alleviate or delay the progression. Although sarcopenia is a disability status that leads to serious health consequences, the scarcity of suitable animal models has curtailed research addressing this disorder. Another limitation in the field of clinical investigation of sarcopenic patients is the lack of a generally accepted definition coupled with the difficulty of adopting common diagnostic criteria. In fact, both do not permit to clarify the exact prevalence rate and consequently limit physicians to establish any kind of therapeutical approach or, when possible, to adopt preventive measures. So, we need more evidence to prove in the future.
Conclusions
Exercise and nutrition supplement can effectively improve the sarcopenia and liver function especially in SMI and albumin level in LC patients.
Acknowledgments
We appreciate the librarian, Ching-Ju Fnag, who provides suggestions on the article searching. We also thank research nurse Chang Hsing-Fen and the partial support from The Chih-Ying Plant R&D Foundation.
Funding: The research was supported in part by
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-639/rc
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-639/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-23-639/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Khatun MS, Biswas MHA. Optimal control strategies for preventing hepatitis B infection and reducing chronic liver cirrhosis incidence. Infect Dis Model 2020;5:91-110. [Crossref] [PubMed]
- Jepsen P, Younossi ZM. The global burden of cirrhosis: A review of disability-adjusted life-years lost and unmet needs. J Hepatol 2021;75:S3-S13. [Crossref] [PubMed]
- Hanai T, Shiraki M, Ohnishi S, et al. Rapid skeletal muscle wasting predicts worse survival in patients with liver cirrhosis. Hepatol Res 2016;46:743-51. [Crossref] [PubMed]
- Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol 2015;49:690-6. [Crossref] [PubMed]
- Bell KE, von Allmen MT, Devries MC, et al. Muscle Disuse as a Pivotal Problem in Sarcopenia-related Muscle Loss and Dysfunction. J Frailty Aging 2016;5:33-41. [PubMed]
- Wu IC, Lin CC, Hsiung CA, et al. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatr Gerontol Int 2014;14:52-60. [Crossref] [PubMed]
- Hanai T, Shiraki M, Nishimura K, et al. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015;31:193-9. [Crossref] [PubMed]
- Kim G, Kang SH, Kim MY, et al. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta-analysis. PLoS One 2017;12:e0186990. [Crossref] [PubMed]
- Bojko M. Causes of Sarcopenia in Liver Cirrhosis. Clin Liver Dis (Hoboken) 2019;14:167-70. [Crossref] [PubMed]
- Sinclair M, Gow PJ, Grossmann M, et al. Review article: sarcopenia in cirrhosis--aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther 2016;43:765-77. [Crossref] [PubMed]
- Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol 2016;65:1232-44. [Crossref] [PubMed]
- Naseer M, Turse EP, Syed A, et al. Interventions to improve sarcopenia in cirrhosis: A systematic review. World J Clin Cases 2019;7:156-70. [Crossref] [PubMed]
- Liao ZY, Chen JL, Xiao MH, et al. The effect of exercise, resveratrol or their combination on Sarcopenia in aged rats via regulation of AMPK/Sirt1 pathway. Exp Gerontol 2017;98:177-83. [Crossref] [PubMed]
- Bémeur C, Butterworth RF. Nutrition in the management of cirrhosis and its neurological complications. J Clin Exp Hepatol 2014;4:141-50. [Crossref] [PubMed]
- Aamann L, Tandon P, Bémeur C. Role of Exercise in the Management of Hepatic Encephalopathy: Experience From Animal and Human Studies. J Clin Exp Hepatol 2019;9:131-6. [Crossref] [PubMed]
- Ruiz-Margáin A, Xie JJ, Román-Calleja BM, et al. Phase Angle From Bioelectrical Impedance for the Assessment of Sarcopenia in Cirrhosis With or Without Ascites. Clin Gastroenterol Hepatol 2021;19:1941-1949.e2. [Crossref] [PubMed]
- Zenith L, Meena N, Ramadi A, et al. Eight weeks of exercise training increases aerobic capacity and muscle mass and reduces fatigue in patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1920-6.e2. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412-23. [Crossref] [PubMed]
- Hiraoka A, Michitaka K, Ueki H, et al. Sarcopenia and two types of presarcopenia in Japanese patients with chronic liver disease. Eur J Gastroenterol Hepatol 2016;28:940-7. [Crossref] [PubMed]
- Nishimura N, Kaji K, Kitagawa K, et al. Intestinal Permeability Is a Mechanical Rheostat in the Pathogenesis of Liver Cirrhosis. Int J Mol Sci 2021;22:6921. [Crossref] [PubMed]
- Trovato FM, Aiello FC, Larocca L, et al. The role of physical activity and nutrition in the sarcopenia of cirrhosis. J Funct Morphol Kinesiol 2016;1:118-25. [Crossref]
- Madden GJ, Petry NM, Badger GJ, et al. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol 1997;5:256-62. [Crossref] [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [Crossref] [PubMed]
- Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc 2020;21:300-307.e2. [Crossref] [PubMed]
- Hernández-Conde M, Llop E, Gómez-Pimpollo L, et al. Adding Branched-Chain Amino Acids to an Enhanced Standard-of-Care Treatment Improves Muscle Mass of Cirrhotic Patients With Sarcopenia: A Placebo-Controlled Trial. Am J Gastroenterol 2021;116:2241-9. [Crossref] [PubMed]
- Macías-Rodríguez RU, Ruiz-Margáin A, Román-Calleja BM, et al. Effect of non-alcoholic beer, diet and exercise on endothelial function, nutrition and quality of life in patients with cirrhosis. World J Hepatol 2020;12:1299-313. [Crossref] [PubMed]
- Lattanzi B, Bruni A, Di Cola S, et al. The Effects of 12-Week Beta-Hydroxy-Beta-Methylbutyrate Supplementation in Patients with Liver Cirrhosis: Results from a Randomized Controlled Single-Blind Pilot Study. Nutrients 2021;13:2296. [Crossref] [PubMed]
- Okubo H, Ando H, Nakadera E, et al. Levocarnitine Supplementation Suppresses Lenvatinib-Related Sarcopenia in Hepatocellular Carcinoma Patients: Results of a Propensity Score Analysis. Nutrients 2021;13:4428. [Crossref] [PubMed]
- Child CG, Turcotte JG. Surgery and portal hypertension. In: Child CG, editor. The liver and portal hypertension. Philadelphia: W. B. Saunders Co.; 1964:50-2.
- Lai JC, Covinsky KE, McCulloch CE, et al. The Liver Frailty Index Improves Mortality Prediction of the Subjective Clinician Assessment in Patients With Cirrhosis. Am J Gastroenterol 2018;113:235-42. [Crossref] [PubMed]
- Wang CW, Lebsack A, Chau S, et al. The Range and Reproducibility of the Liver Frailty Index. Liver Transpl 2019;25:841-7. [Crossref] [PubMed]
- Cawthon PM. Assessment of Lean Mass and Physical Performance in Sarcopenia. J Clin Densitom 2015;18:467-71. [Crossref] [PubMed]
- Lai JC, Covinsky KE, Dodge JL, et al. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017;66:564-74. [Crossref] [PubMed]
- Hirota K, Kawaguchi T, Koya S, et al. Clinical utility of the Liver Frailty Index for predicting muscle atrophy in chronic liver disease patients with hepatocellular carcinoma. Hepatol Res 2020;50:330-41. [Crossref] [PubMed]
- Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013;93:993-1017. [Crossref] [PubMed]
- Chen SH, Liao WC, Huang AC. The effect of exercise on myostatin. Journal of Chiao Da Physical Education 2013;5:41-9.
- Liao CD, Chen HC, Kuo YC, et al. Effects of Muscle Strength Training on Muscle Mass Gain and Hypertrophy in Older Adults With Osteoarthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res (Hoboken) 2020;72:1703-18. [Crossref] [PubMed]
- Soldera J, Rech A, Rossi D, et al. Resistance training effects on muscle strength and muscle mass in compensated cirrhotic patients: preliminary results from a randomized controlled trial. United European Gastroenterol J 2020;8:619-20.
- Aamann L, Dam G, Rinnov AR, et al. Physical exercise for people with cirrhosis. Cochrane Database Syst Rev 2018;12:CD012678. [PubMed]
- Narao H, Hirota K, Koya S, et al. Effects of In-Hospital Physical Therapy on Activities of Daily Living in Patients with Hepatocellular Carcinoma. Int J Environ Res Public Health 2020;17:9098. [Crossref] [PubMed]
- Tsuchihashi J, Koya S, Hirota K, et al. Effects of In-Hospital Exercise on Frailty in Patients with Hepatocellular Carcinoma. Cancers (Basel) 2021;13:194. [Crossref] [PubMed]
- Hashida R, Kawaguchi T, Koya S, et al. Impact of cancer rehabilitation on the prognosis of patients with hepatocellular carcinoma. Oncol Lett 2020;19:2355-67. [Crossref] [PubMed]
- Koya S, Kawaguchi T, Hashida R, et al. Effects of in-hospital exercise on sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol 2019;34:580-8. [Crossref] [PubMed]
- Koya S, Kawaguchi T, Hashida R, et al. Effects of in-hospital exercise on liver function, physical ability, and muscle mass during treatment of hepatoma in patients with chronic liver disease. Hepatol Res 2017;47:E22-34. [Crossref] [PubMed]
- Plauth M, Bernal W, Dasarathy S, et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr 2019;38:485-521. [Crossref] [PubMed]
- Yamato M, Muto Y, Yoshida T, et al. Clearance rate of plasma branched-chain amino acids correlates significantly with blood ammonia level in patients with liver cirrhosis. International Hepatology Communications 1995;3:91-6. [Crossref]
- Steigmann F, Szanto PB, Poulos A, et al. Significance of serum aminograms in diagnosis and prognosis of liver diseases. J Clin Gastroenterol 1984;6:453-60. [Crossref] [PubMed]
- Kawaguchi T, Izumi N, Charlton MR, et al. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011;54:1063-70. [Crossref] [PubMed]
- Kawaguchi T, Shiraishi K, Ito T, et al. Branched-chain amino acids prevent hepatocarcinogenesis and prolong survival of patients with cirrhosis. Clin Gastroenterol Hepatol 2014;12:1012-8.e1. [Crossref] [PubMed]
- Dattola A, Silvestri M, Bennardo L, et al. Role of Vitamins in Skin Health: a Systematic Review. Curr Nutr Rep 2020;9:226-35. [Crossref] [PubMed]
- Sánchez-Muniz FJ, Macho-González A, Garcimartín A, et al. The Nutritional Components of Beer and Its Relationship with Neurodegeneration and Alzheimer's Disease. Nutrients 2019;11:1558. [Crossref] [PubMed]
- Kawaguchi T, Tsutsumi T, Nakano D, et al. MAFLD: Renovation of clinical practice and disease awareness of fatty liver. Hepatol Res 2022;52:422-32. [Crossref] [PubMed]
- Fukunaga S, Mukasa M, Nakane T, et al. Impact of non-obese metabolic dysfunction-associated fatty liver disease on risk factors for the recurrence of esophageal squamous cell carcinoma treated with endoscopic submucosal dissection: A multicenter study. Hepatol Res 2024;54:201-12. [Crossref] [PubMed]
- Eslam M, Sanyal AJ, George J, et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020;158:1999-2014.e1. [Crossref] [PubMed]
- Inamine S, Kage M, Akiba J, et al. Metabolic dysfunction-associated fatty liver disease directly related to liver fibrosis independent of insulin resistance, hyperlipidemia, and alcohol intake in morbidly obese patients. Hepatol Res 2022;52:841-58. [Crossref] [PubMed]
- Sano T, Amano K, Ide T, et al. Metabolic management after sustained virologic response in elderly patients with hepatitis C virus: A multicenter study. Hepatol Res 2024;54:326-35. [Crossref] [PubMed]
- Tsutsumi T, Eslam M, Kawaguchi T, et al. MAFLD better predicts the progression of atherosclerotic cardiovascular risk than NAFLD: Generalized estimating equation approach. Hepatol Res 2021;51:1115-28. [Crossref] [PubMed]
- Kobayashi T, Iwaki M, Nogami A, et al. Prediction of outcomes in patients with metabolic dysfunction-associated steatotic liver disease based on initial measurements and subsequent changes in magnetic resonance elastography. J Gastroenterol 2024;59:56-65. [Crossref] [PubMed]
- Iwaki M, Fujii H, Hayashi H, et al. Prognosis of biopsy-confirmed metabolic dysfunction- associated steatotic liver disease: A sub-analysis of the CLIONE study. Clin Mol Hepatol 2024;30:225-34. [Crossref] [PubMed]
- Kim HK, Bae SJ, Lee MJ, et al. Association of Visceral Fat Obesity, Sarcopenia, and Myosteatosis with Non-Alcoholic Fatty Liver Disease without Obesity. Clin Mol Hepatol 2023;29:987-1001. [Crossref] [PubMed]
- Sinn DH, Kang D, Kang M, et al. Nonalcoholic fatty liver disease and accelerated loss of skeletal muscle mass: A longitudinal cohort study. Hepatology 2022;76:1746-54. [Crossref] [PubMed]
- Koo BK, Kim D, Joo SK, et al. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J Hepatol 2017;66:123-31. [Crossref] [PubMed]
- Harring M, Golabi P, Paik JM, et al. Sarcopenia Among Patients With Nonalcoholic Fatty Liver Disease (NAFLD) Is Associated With Advanced Fibrosis. Clin Gastroenterol Hepatol 2023;21:2876-2888.e5. [Crossref] [PubMed]
- Kim D, Wijarnpreecha K, Sandhu KK, et al. Sarcopenia in nonalcoholic fatty liver disease and all-cause and cause-specific mortality in the United States. Liver Int 2021;41:1832-40. [Crossref] [PubMed]
- Tandon P, Montano-Loza AJ, Lai JC, et al. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol 2021;75:S147-62. [Crossref] [PubMed]
- European Association for the Study of the Liver (EASL). European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388-402. [Crossref] [PubMed]
- Dent E, Morley JE, Cruz-Jentoft AJ, et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J Nutr Health Aging 2018;22:1148-61. [Crossref] [PubMed]
- Zhao X, Shi X, Gu H, et al. Association between handgrip strength, nonalcoholic fatty liver disease, advanced hepatic fibrosis and its modifiers: Evidence from the NHANES database of the USA. J Gastroenterol Hepatol 2023;38:1734-42. [Crossref] [PubMed]
- Mertz KH, Reitelseder S, Bechshoeft R, et al. The effect of daily protein supplementation, with or without resistance training for 1 year, on muscle size, strength, and function in healthy older adults: A randomized controlled trial. Am J Clin Nutr 2021;113:790-800. [Crossref] [PubMed]
- Bischoff SC, Bernal W, Dasarathy S, et al. ESPEN practical guideline: Clinical nutrition in liver disease. Clin Nutr 2020;39:3533-62. [Crossref] [PubMed]
- Papadopoulou SK, Detopoulou P, Voulgaridou G, et al. Mediterranean Diet and Sarcopenia Features in Apparently Healthy Adults over 65 Years: A Systematic Review. Nutrients 2023;15:1104. [Crossref] [PubMed]
- Tarantino G, Sinatti G, Citro V, et al. Sarcopenia, a condition shared by various diseases: can we alleviate or delay the progression? Intern Emerg Med 2023;18:1887-95. [Crossref] [PubMed]



