
Trimming the fat myth: adipose tissue’s role in liver regeneration
We read with great interest the paper from Jena (Germany) recently published in Hepatobiliary Surgery and Nutrition by Deeb et al., which caught our attention, given its clinical relevance. In fact, post-hepatectomy liver failure (PHLF) remains nowadays a significant clinical challenge, occasionally leading to death or potentially requiring, in highly selected cases, rescue liver transplantation (1). Within quaternary care centers, the incidence of PHLF fluctuates from 5% to 20%, depending on the criteria employed, the underlying liver parenchyma quality and the type of hepatectomy (2). Hence, liver regeneration (LR) holds paramount importance in hepatic surgery as it directly impacts post-operative outcomes (3): it is a strictly orchestrated phenomenon that entails the activation of hepatocytes and hepatic progenitor cells, as well as the regulation of cell-cycle genes and growth factors. Features contributing to PHLF include pre-operative, intra-operative and post-operative elements, leading to careful patient selection (4). The signaling pathways involved in LR are not yet fully understood, and still hold potential for understanding and treating liver disease. Therefore, exploring the molecular processes that drive efficient LR after liver resection constitutes a pivotal research endeavor. The early stage of LR, known as the priming phase, which occurs from 0 to 5 hours after partial hepatectomy (PH), is initiated by rapid haemodynamic changes (5) that result in the stress-induced release of various humoral factors from the liver tissue, such as epidermal growth factor (EGF), hepatocyte growth factor (HGF), transforming growth factor-α (TGF-α), interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α) and nitric oxide (NO) (6,7). This phase precedes the proliferation phase, which lasts from day 1 to day 7 after PH. In addition, the role of specific hepatokines and growth factors in initiating cellular signaling is critical, activating a well-coordinated genetic expression programme that oversees the step-by-step remodeling of the extracellular matrix, cellular proliferation and liver growth of the proliferation and termination phases (up to 12 weeks) (8). All these factors are intricately involved in a complex network that regulates the regenerative process of the liver after hepatectomy, contributing in fine to the almost ad integrum restoration of hepatic mass and function.
Adipose tissue, primarily known as “simple” energy store, has recently emerged to be involved in a variety of physiological and pathological processes, including wound healing and tissue regeneration. Furthermore, as most of the synthesis and utilization of lipid acids occurs in the liver, lipid metabolism has been shown to play an important role in LR in several animal models (9,10).
Adipose tissue metabolism affects LR mainly through two distinct mechanisms:
- By mobilizing free fatty acids from extra-hepatic adipose tissue, with systemic hypoglycemia and consequent transient liver steatosis (11) (Figure 1).
- By activating the mobilization of adipose-derived mesenchymal stem cells (AD-MSCs) from fat store (12) (Figure 2).
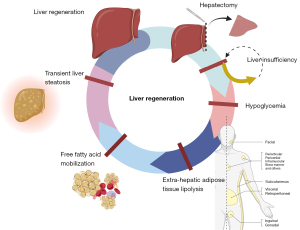
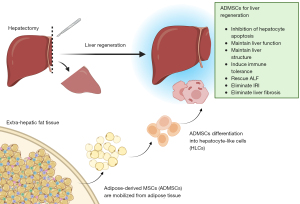
Notwithstanding recent improved insights into LR, the prevention of PHLF has seen little progress, with traditional approaches such as limiting chemotherapy, inducing pre-resectional liver hypertrophy and managing portal hypertension having limited success in reducing the PHLF incidence. There is an urgent need for new, impactful hypotheses.
The study by Deeb and colleagues (13) described their single institution’s experience evaluating the impact of subcutaneous adipose tissue in LR. The study involved a cohort of 120 patients, including 70 living donors undergoing right hepatectomy and 50 right liver graft transplant recipients between 2013 and 2022. Liver volumetry and body fat analysis were assessed by computed tomography (CT) and elaborated using specialized software (Synapse3D, FUMIFILM). The analysis was based on Hounsfield unit values to differentiate adipose tissue characteristics and distribution. LR was assessed at three different time points: pre-surgery, within 14 days of resection and 6 months after surgery. Liver volumetric changes were quantified using the future liver volume (FLV). Additionally, psoas volume was assessed as a secondary factor related to liver recovery. The inclusion of this parameter in the multivariate statistical analysis provided a comprehensive understanding of the factors influencing postoperative liver recovery. Results showed a significant correlation between subcutaneous fat mass index (sFMI) and liver volume gain, both short term (r=0.173, P=0.030) and long term (r=0.395, P=0.0004). Noteworthy, this positive effect was found to be significant only in the donor group, highlighting the unique role of subcutaneous adipose tissue in the regenerative process following liver resection in living donors.
Furthermore, as shown by the correlation between the ratio of visceral to subcutaneous fat mass (vFMI/sFMI) and liver volume gain, a negative effect of visceral adipose tissue on LR was observed. These observations suggest the role of subcutaneous adipose tissue as a positive predictive factor for estimating the regenerative capacity of the liver after PH; this evidence might be of crucial importance in the pre-operative assessment and might have an impact on the selection of living donors for liver transplantation. Furthermore, the study suggests that nutritional assessment and supplementation, focusing on subcutaneous fat accumulation prior to liver surgery, could positively impact LR. These findings highlight the importance of subcutaneous fat in LR, particularly in the context of living-donor liver transplant. This observation is crucial as it opens up for further research into the mechanisms underlying this relationship and the potential for therapeutic interventions aimed at improving LR in the surgical setting. We could, for example, imagine leaving a small volume of remnant liver in donors with significant subcutaneous fat, thus predicting their high regeneration capacity. This would enable donors/recipients matches that were previously contra-indicated.
While presenting several limitations, these findings could drive interesting food for thought for future trials such as more-strict time criteria for volumetric measurements and a more consistent method to better assess liver function after surgery.
One initial concern is the observation that only donors showed a statistical correlation. This could be attributed to the compromised health and nutritional status of recipients. While increased fat intake may enhance LR, the overall poor general status of the recipients may counteract this beneficial effect. Furthermore, no cases of PHLF were observed in the recipient patient group. This finding could be retrospectively related to an insufficient subcutaneous fat reserve, a factor that could potentially have been predicted. Another major concern relates to the selection of eligible patients as donors. The authors highlighted an inherent selection bias by only considering patients with maximum body mass index (BMI) of 34.1 kg/m2 (class 1 obesity). The scientific literature agree that obesity classed as 1 or 2 (BMI 35 to 40 kg/m2) should be considered as a contraindication for liver donation or a risk factor for hepatic resections. This is due to the presence of liver graft steatosis and a well-documented higher perioperative morbidity and mortality for class 1 or 2 obesity compared to normal or overweight patients. In extreme cases, a low-calorie, high-protein diet is even suggested to lose weight and ‘cleanse’ the hepatic steatosis (14). Weight loss would be associated with a reduction in subcutaneous (and/or visceral?) fat and consequently reduced ‘support’ during LR. Finally, the correlation between subcutaneous fat and LR, although significant, is weak (r<0.6).
In conclusion, this study provides compelling evidence for the beneficial effect of subcutaneous adipose tissue on LR following PH in living donors. This improves our understanding of the factors influencing LR and offers potential strategies to optimize outcomes of liver surgery not only in oncological settings but also for transplantation. These results also provide important anticipatory guidance for the living donor liver transplantation (LDLT) community including the management of LDLT high-BMI donor selection. Forthcoming prospective studies are needed to further clarify the protective effect of subcutaneous adipose tissue in liver surgery and transplantation, including investigations into the histological and functional aspects of the regenerated liver (15).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-181/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Azoulay D, Desterke C, Bhangui P, et al. Rescue Liver Transplantation for Posthepatectomy Liver Failure: A Systematic Review and Survey of an International Experience. Transplantation 2024;108:947-57. [Crossref] [PubMed]
- Sparrelid E, Olthof PB, Dasari BVM, et al. Current evidence on posthepatectomy liver failure: comprehensive review. BJS Open 2022;6:zrac142. [Crossref] [PubMed]
- Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2-13. [Crossref] [PubMed]
- Garcea G, Maddern GJ. Liver failure after major hepatic resection. J Hepatobiliary Pancreat Surg 2009;16:145-55. [Crossref] [PubMed]
- Murtha-Lemekhova A, Fuchs J, Ghamarnejad O, et al. Influence of cytokines, circulating markers and growth factors on liver regeneration and post-hepatectomy liver failure: a systematic review and meta-analysis. Sci Rep 2021;11:13739. [Crossref] [PubMed]
- Abu Rmilah AA, Zhou W, Nyberg SL. Hormonal Contribution to Liver Regeneration. Mayo Clin Proc Innov Qual Outcomes 2020;4:315-38. [Crossref] [PubMed]
- Gonzales E, Julien B, Serrière-Lanneau V, et al. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol 2010;52:54-62. [Crossref] [PubMed]
- Wang HH, Lautt WW. Evidence of nitric oxide, a flow-dependent factor, being a trigger of liver regeneration in rats. Can J Physiol Pharmacol 1998;76:1072-9. [Crossref] [PubMed]
- Zhou X, Huang G, Wang L, et al. L-carnitine promotes liver regeneration after hepatectomy by enhancing lipid metabolism. J Transl Med 2023;21:487. [Crossref] [PubMed]
- Fazel Modares N, Polz R, Haghighi F, et al. IL-6 Trans-signaling Controls Liver Regeneration After Partial Hepatectomy. Hepatology 2019;70:2075-91. [Crossref] [PubMed]
- Thevananther S. Adipose to the rescue: peripheral fat fuels liver regeneration. Hepatology 2010;52:1875-6. [Crossref] [PubMed]
- Yang D, Wang ZQ, Deng JQ, et al. Adipose-derived stem cells: A candidate for liver regeneration. J Dig Dis 2015;16:489-98. [Crossref] [PubMed]
- Deeb AA, Settmacher U, Ardelt M, et al. Adipose tissue induces a better liver regeneration after living liver donation in normal weight donors. Hepatobiliary Surg Nutr 2023;12:341-50. [Crossref] [PubMed]
- Peloso A, Tihy M, Moeckli B, et al. Clearing Steatosis Prior to Liver Surgery for Colorectal Metastasis: A Narrative Review and Case Illustration. Nutrients 2022;14:5340. [Crossref] [PubMed]
- Lucey MR, Furuya KN, Foley DP. Liver Transplantation. N Engl J Med 2023;389:1888-900. [Crossref] [PubMed]


