
Long-term normothermic machine perfusion of fatty livers: towards transplanting untransplantable livers?
Since the very early days of clinical liver transplantation (LT), transplant surgeons and professionals have been confronted with the increased risk of failure associated with the use of fatty liver grafts (1). Notwithstanding the wide variability in steatosis assessment across different centers and pathologists (2), utilization of livers with moderate (≥30%) or severe (≥60%) macrovesicular steatosis has been consistently associated with an increased risk of primary non-function, early allograft dysfunction, acute kidney injury, as well as inferior graft and patient survival (3). The mechanisms behind the increased susceptibility of steatotic livers to ischemia-reperfusion injury (IRI) are multiple, including disturbances to microcirculation due to sinusoidal narrowing, increased oxidative stress upon reperfusion and enhanced lipid peroxidation, leading to an increased release of inflammatory mediators like IL6, IL1β and so-called damage associated molecular patterns (DAMP), like cell-free DNA and mitochondrial DNA. Histologically, this is reflected by hepatocyte death by necrosis rather than apoptosis, pseudopeliotic steatosis (i.e., the expulsion of lipid droplets into the extracellular space) and lately, by tissue remodelling and fibrosis (4,5). Consequently, steatosis represents one frequent reason for graft non-utilization. As the prevalence of metabolic associated steatotic liver disease is projected to rise in the population and in organ donors, developing mitigation strategies against IRI in fatty livers is one focus of basic science and clinical research in LT.
Machine perfusion has been reappraised in LT to improve preservation of livers from extended-criteria donors, including those bearing significant macrosteatosis. In this setting, outcomes of hypothermic oxygenated machine perfusion (HOPE) have been controversial (6-8), also due to the lack of the possibility to properly assess their viability before transplant. Normothermic machine perfusion (NMP), by which the liver is maintained in a physiological environment and provided with oxygen and nutrients, could represent the ideal preservation technique for fatty livers, as a platform simultaneously allowing better preservation, viability testing, and administering organ therapeutics. However, especially when NMP is applied after a period of cold storage, steatotic livers frequently fail to meet criteria for transplantation and, even when they do, primary non-function is not ruled out (9).
Recently, Sousa da Silva et al. (10) published a paper reporting on the possibility of defatting livers during long-term NMP. In this series, they used the arguably most advanced NMP device available nowadays—the Wyss machine (11)—incorporating several core technologies, namely a dialysis unit, an inflatable mat mimicking diaphragmatic movements, an artificial pancreas, and a close monitoring of nutrition and vasopressors requirements. Twenty steatotic livers were perfused for an average time of one week and up to 12 days, of which 14 were whole livers having been discarded for LT and 6 were specimen of hemihepatectomy performed for an oncological indication. During NMP, 10 livers (“responders”) displayed a significant loss of fat, which was almost complete in 5 cases, whereas 10 livers (“non-responders”) did not. Authors claim that more efficient defatting was linked to the progressive adaptations that were made to their protocol, namely avoiding glucose supplementation to minimize lipogenesis, maintaining normoglycemia by continuous adjustment of glucagon and insulin infusions, administering nutrition according to circadian cycles, and supplementing the perfusate with L-carnitine (promoter of the transport of fatty acids into mitochondria) and fenofibrate (promoter of β-oxidation). Interestingly, Authors noticed a histological pattern similar to what is observed in-vivo: in responders, fat droplets were mostly intracellular, whereas non-responders developed pseudopeliotic steatosis, which was associated with a more pronounced activation of Kupffer and stellate cells. Authors are to be congratulated for successfully achieving what appears to be a prohibitive task, i.e., developing a device allowing semi-automated long-term NMP, which is the necessary prerequisite to study complex biological processes, including but not limited to liver defatting.
At present, two philosophies drive the application of NMP in fatty livers. One approach is using NMP as a platform to cure the disease, i.e., steatosis, based on the assumption that clearing the liver from fat accumulation will result in improved outcomes after LT. The study by Sousa da Silva et al. (10) comes in the wake of previous attempts at achieving defatting of discarded human livers during shorter NMP by supplementing the perfusate with defatting agents (12) and/or by incorporating a lipid apheresis filter in the circuit (13), which were also marked by some success. However, while implementation of a defatting protocol has been generally associated with improved viability parameters on the machine, no data on livers treated by a defatting protocol and then transplanted are available. The Oxford group is currently recruiting patients for a randomized clinical trial on liver defatting, with preliminary results anticipated in 2025 (ISRCTN14957538).
Some limitations of the Sousa da Silva et al. (10) paper have to be acknowledged, namely the study design and the high heterogeneity of the included livers, possibly representing a source of confounding. Furthermore, it is not possible to establish a causal relationship between successful defatting and the protocol modifications that were progressively implemented. More substantially, the protocol applied by the Authors appears to be labour and technology intensive, which limits its wide applicability in clinical settings that are usually already strained by workforce shortage. A further issue is represented by how the viability of these long-perfused livers should be assessed. Authors stated that all livers were viable during long-term NMP, so arguably (and provocatively) all these livers could have been transplanted despite not achieving successful defatting. In our experience, viability assessment of livers with ≥30% macrovesicular steatosis has proven to be problematic in some cases (9). While some degree of deviance from most widely adopted viability criteria can be accepted in particular scenarios (i.e., standard grafts undergoing NMP for logistical reasons), signs of impending poor function after LT can be very subtle in fatty grafts, suggesting extreme caution.
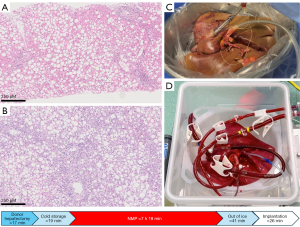
A second approach is represented by trying to minimize IRI enabling transplantation of these grafts which, most frequently, exhibit perfectly normal function in the donor. As the main issue with steatotic grafts is initial poor function (3), improving functional recovery after LT would allow steatosis to be cleared in-vivo in the recipient, similarly to what Sousa da Silva et al. have shown to happen on their long-term NMP device (Figure 1) (10). An appealing approach to improve preservation of steatotic livers is represented by upfront NMP, or so-called normothermic machine preservation, which incorporates the concept of minimization of initial cold ischemia time. The rationale for this approach is based on the fact that preclinical models have shown that even a short period of cold preservation is detrimental in severely damaged grafts undergoing NMP and that, due to their high content in fat, steatotic livers are particularly prone to damage when they are cooled down. This prompted our group to implement upfront NMP when significant graft steatosis is expected based on donor data. In a proof-of-concept case, we have shown that even grafts with severe macrovesicular steatosis can be successfully transplanted after assessment and preservation by upfront NMP (14). A message drawn from our experience is that minimizing pre- and post-NMP ischemia time and avoiding graft cooling are key elements in allowing successful recovery of these extremely marginal grafts (Figure 2). Pushing this concept further, the Guangzhou group developed a protocol for ischemia-free LT, in which NMP is initiated in the donor and continued until implantation into the recipient (15). Interestingly, this protocol was first applied to a liver with 50% macrovesicular steatosis. Although interesting, ischemia-free LT is rather complex, and its application has remained limited to the developing center. More importantly, any upfront approach relies on the correct identification of those graft that might benefit from these expensive and logistically demanding procedures. Unfortunately, prediction of liver steatosis based on donor data is highly inaccurate and, unless a pre-procurement biopsy is available, establishing the indication for upfront NMP or ischemia-free LT remains difficult.

In conclusion, despite recent advances in organ procurement and preservation, achieving successful transplantation of fatty liver grafts appears to remain an unresolved issue. Although the Sousa da Silva et al.’s paper (10) sheds some new light on this complex topic, further studies are necessary to confirm that successful defatting translates into good graft function after LT. If successful, application of such a strategy will likely require concentrating the necessary expertise in organ perfusion hubs, with dedicated surgeons, perfusionists and engineers. At present, the possibilities offered by long-term NMP appear appealing mainly for basic science and pre-clinical research, as a platform to study IRI, liver metabolism and other biological processes, similar to what has been done for more than a century with different isolated-perfused liver models.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, HepatoBiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-285/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Todo S, Demetris AJ, Makowka L, et al. Primary nonfunction of hepatic allografts with preexisting fatty infiltration. Transplantation 1989;47:903-5. [Crossref] [PubMed]
- Gambella A, Salvi M, Molinaro L, et al. Improved assessment of donor liver steatosis using Banff consensus recommendations and deep learning algorithms. J Hepatol 2024;80:495-504. [Crossref] [PubMed]
- Croome KP, Lee DD, Taner CB. The "Skinny" on Assessment and Utilization of Steatotic Liver Grafts: A Systematic Review. Liver Transpl 2019;25:488-99. [Crossref] [PubMed]
- Abbas SH, Ceresa CDL, Pollok JM. Steatotic Donor Transplant Livers: Preservation Strategies to Mitigate against Ischaemia-Reperfusion Injury. Int J Mol Sci 2024;25:4648. [Crossref] [PubMed]
- Patrono D, De Stefano N, Vissio E, et al. How to Preserve Steatotic Liver Grafts for Transplantation. J Clin Med 2023;12:3982. [Crossref] [PubMed]
- Kron P, Schlegel A, Mancina L, et al. Hypothermic oxygenated perfusion (HOPE) for fatty liver grafts in rats and humans. J Hepatol 2017;S0168-8278(17)32268-7.
- Patrono D, Catalano G, Rizza G, et al. Perfusate Analysis During Dual Hypothermic Oxygenated Machine Perfusion of Liver Grafts: Correlations With Donor Factors and Early Outcomes. Transplantation 2020;104:1929-42. [Crossref] [PubMed]
- Patrono D, Cussa D, Sciannameo V, et al. Outcome of liver transplantation with grafts from brain-dead donors treated with dual hypothermic oxygenated machine perfusion, with particular reference to elderly donors. Am J Transplant 2022;22:1382-95. [Crossref] [PubMed]
- Patrono D, De Carlis R, Gambella A, et al. Viability assessment and transplantation of fatty liver grafts using end-ischemic normothermic machine perfusion. Liver Transpl 2023;29:508-20. [Crossref] [PubMed]
- Sousa Da Silva RX, Bautista Borrego L, Lenggenhager D, et al. Defatting of Human Livers During Long-Term e x situ Normothermic Perfusion: Novel Strategy to Rescue Discarded Organs for Transplantation. Ann Surg 2023;278:669-75. [Crossref] [PubMed]
- Eshmuminov D, Becker D, Bautista Borrego L, et al. An integrated perfusion machine preserves injured human livers for 1 week. Nat Biotechnol 2020;38:189-98. [Crossref] [PubMed]
- Boteon YL, Attard J, Boteon APCS, et al. Manipulation of Lipid Metabolism During Normothermic Machine Perfusion: Effect of Defatting Therapies on Donor Liver Functional Recovery. Liver Transpl 2019;25:1007-22. [Crossref] [PubMed]
- Ceresa CDL, Nasralla D, Pollok JM, et al. Machine perfusion of the liver: applications in transplantation and beyond. Nat Rev Gastroenterol Hepatol 2022;19:199-209. [Crossref] [PubMed]
- Patrono D, Apostu AL, Rizza G, et al. Upfront Normothermic Machine Perfusion for a Liver Graft with Severe Macrovesicular Steatosis: A Proof-of-Concept Case. Transplantology 2023;4:151-60. [Crossref]
- Guo Z, Zhao Q, Jia Z, et al. A randomized-controlled trial of ischemia-free liver transplantation for end-stage liver disease. J Hepatol 2023;79:394-402. [Crossref] [PubMed]


