
Deciphering molecular crosstalk mechanisms between skeletal muscle atrophy and KRAS-mutant pancreatic cancer: a literature review
Introduction
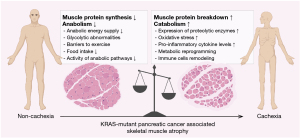
Cachexia, a significant complication in cancer progression, was first characterized by Evans et al. (1) as a syndrome of “consuming without gaining weight”, primarily manifested by a reduction in skeletal muscle mass. This syndrome is defined by involuntary weight loss due to muscle atrophy, which is non-responsive to nutritional supplementation and resistant to pharmacological treatments. Over the past decade, the impact of cachexia on the efficacy of multidrug chemotherapy regimens has become increasingly evident (2,3). Concurrently, the conceptualization and diagnostic criteria for cachexia have evolved. Initially, the 2011 Fearon criteria focused on weight loss and body mass index (BMI), where the main criterion for cachexia is weight loss >5% over past 6 months (in absence of simple starvation) (4). Subsequent revisions have incorporated physical performance and other measurable indicators (5). The latest definition by the European Society of Medical Oncology (ESMO) in 2021 categorizes cachexia as malnutrition caused by disease-related systemic inflammation (6). This is further refined in the 2020 Global Leadership Initiative on Malnutrition (GLIM) criteria, which require a positive screening result and at least one phenotypic criterion, such as weight loss, low body mass, or diminished muscle mass, for a malnutrition diagnosis (7).
In the realm of pancreatic cancer (PC), cachexia is ubiquitously observed throughout all disease stages (8). Notably, about 80% of individuals diagnosed with pancreatic ductal adenocarcinoma (PDAC) endure cachexia at some phase of their disease, representing the highest incidence among all cancers (9) (Figure 1). This underscores the critical need to integrate cachexia management within the therapeutic strategies for PC. Although criteria for weight loss and low body mass have been clearly defined, consensus on defining “reduced muscle mass” continues to be ambiguous. Existing diagnostic metrics, such as the sex-specific L3 vertebrae skeletal muscle index, mid-upper arm muscle area, and appendicular skeletal muscle index, predominantly assess sarcopenia (4,10-14).
Nonetheless, advancing our understanding of the molecular mechanisms underlying muscle wasting in PC may facilitate the development of more targeted biomarkers, thereby improving both diagnosis and treatment of PC-associated cachexia. We present this article in accordance with the Narrative Review reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-282/rc).
Methods
We searched for literature with terms “Cachexia/Sarcopenia”, “Skeletal Muscle Wasting/Atrophy”, “Pancreatic Cancer”, and “KRAS Mutation” published in PubMed, Web of Science, Google Scholar and other search engines, encompassing articles in the English language up to May 21st, 2024. Studies on PC models and patients were included. For detailed information, please refer to the search strategy summary in Table 1.
Table 1
| Items | Specification |
|---|---|
| Date of search | The literature search was conducted on May 21, 2024 |
| Databases and other sources searched | PubMed, Web of Science, Google Scholar. Additional searches were conducted using relevant search engines to ensure comprehensive coverage |
| Search terms used | A combination of MeSH terms and free-text keywords was employed, including: “Cachexia/Sarcopenia”, “Skeletal Muscle Wasting/Atrophy”, “Pancreatic Cancer”, and “KRAS Mutation” |
| Timeframe | The search covered studies published up to May 2024 |
| Inclusion and exclusion criteria | Inclusion: studies involving pancreatic cancer models or patients, with a focus on KRAS mutations |
| Exclusion: case reports or studies lacking relevance to the molecular mechanisms linking pancreatic cancer and muscle wasting | |
| Selection process | Two independent reviewers screened the studies, with disagreements resolved by consensus. Only studies meeting the predefined inclusion criteria were selected |
KRAS, Kirsten rat sarcoma viral oncogene homologue.
Molecular crosstalk mechanisms between skeletal muscle atrophy and Kirsten rat sarcoma viral oncogene homologue (KRAS)-mutant PC
KRAS-mutant PC mediates depletion of muscle energy reserves and energy reallocation
Cachexia, a defining feature of PC, is characterized by a metabolic shift favoring catabolism over anabolism, which fosters a state of chronic inflammation. This metabolic alteration impairs muscle protein synthesis, enhances proteolysis, and accelerates myofiber degradation. Notably, oncogenic modifications in PC, particularly KRAS mutations, drive a systemic metabolic reprogramming that supports both tumor proliferation and cachectic muscle wasting (Figure 1).
KRAS mutation, in conjunction with metabolic disturbances such as insulin resistance, plays a critical role in PC pathophysiology. Insulin resistance increases the risk of developing PDAC and has been shown to be mitigated by anti-diabetic treatments (15,16). Furthermore, the interaction between KRAS mutation and a hyperglycemic environment creates a self-sustaining cycle conducive to both tumor growth and muscle wasting. This mutation enhances glycolytic flux by upregulating key enzymes, and elevated glucose levels lead to pancreas-specific DNA damage, further accelerating the mutation process (17,18). In cells with KRAS mutations, increased glucose uptake via glucose transporters (GLUTs) and heightened glycolysis provides intermediates essential for biosynthetic pathways (19).
Moreover, the pentose phosphate pathway (PPP) serves a dual role in PC cells by generating nicotinamide adenine dinucleotide phosphate (NADPH) and ribose bases, vital for nucleotide synthesis and maintaining redox balance. PC cells also exhibit the Warburg effect, a metabolic phenomenon where glucose is predominantly converted to pyruvate and lactate in the cytosol under anaerobic conditions. This increased glycolytic activity leads to enhanced glucose uptake and lactate secretion, contributing to the systemic energy depletion characteristic of cancer cachexia (20,21).
In addition, PC cells exploit alternative carbon sources to fuel the tricarboxylic acid (TCA) cycle, such as glutamine and aspartate, both of which are integral to maintaining skeletal muscle mass (22). Glutamine importation through solute carrier family 1 member 5 (SLC1A5) and its transformation to glutamate by glutaminase 1 (GLS1) fuel the TCA cycle (23), emphasizing glutamine’s role in energy production. Inhibiting glutamine transport by downregulating its transporter SLC1A5 has been shown to attenuate weight loss in PC, albeit without impeding tumor growth (24). Conversely, obstructing aspartate transportation into mitochondria through UCP2 transporter diminishes PDAC cell growth (25). This underscores the versatility of PC in utilizing diverse nutritional sources, often at the expense of body and muscle mass. KRAS mutations further reprogram metabolism by channeling TCA cycle intermediates via malic enzyme 1 (ME1) to produce NADPH and amino acids, enhancing cellular antioxidant capacity and providing synthesis substrates. Simultaneously, KRAS mutations drive fatty acid oxidation (FAO) for energy and NADPH production (26,27), underscoring the metabolic flexibility of cancer cells to meet their energy needs. Collectively, metabolic reprogramming by PC leads to energy extraction from skeletal muscle myotubes. This streamlined overview encapsulates the metabolic intricacies associated with KRAS-driven PC, spotlighting the interplay between tumor growth and host energy metabolism (Table 2).
Table 2
| Origin | Potential biomarkers |
|---|---|
| PC cachexia induction factors | MURF-1, Atrogin-1, myostatin, GDF15, GLUT4, Activin A, PAUF, IRS-1, Ang II, PTHrP, SIRT1/NOX4 |
| Systematic inflammation and immunity factors | PLR, CRP, MyD88 |
| Activators of NF-κB signaling: TNF-α, IL-1β, IL-6, MCP-1/CCL2, TWEAK, PTX3, OSMR/EDA2R/NIK | |
| Immune cells and related markers: M2-like macrophages, Ly6G+ neutrophils and granulocytic MDSCs, NLR, LCN2, Cathepsin B | |
| Muscle and lipid wasting products | Fat wasting: ATGL, HSL, UCP1, LCN2, ZAG, C18:C24 ceramide ratio |
| Skeletal muscle wasting: CNDP1, β-dystroglycan | |
| Non-coding RNAs | miR-21, miR-155, miR-let-7b-5p, miR-373, miR-30b/c, miR-494-3p, miR-9, miR-338-3p, miR-106b, miR-93, miR-27b |
| Extra-cellular matrix factors | TIMP-1 |
KRAS, Kirsten rat sarcoma viral oncogene homologue; PC, pancreatic cancer; MURF-1, muscle RING-finger protein-1; GDF15, growth differentiation factor 15; GLUT4, glucose transporter 4; PAUF, pancreatic adenocarcinoma upregulated factor; IRS-1, insulin receptor substrate 1; Ang II, angiotensin II; PTHrP, parathyroid hormone-related protein; SIRT1, Sirtuin1; PLR, platelet-lymphocyte ratio; CRP, C-reactive protein; MyD88, myeloid differentiation primary response gene 88 protein; NF-κB, nuclear factor kappa B; TNF-α, tumor necrosis factor alpha; IL-1β, interleukin 1 beta; IL-6, interleukin 6; MCP-1, monocyte chemoattractant protein 1; CCL2, CC-motif chemokine ligand 2; TWEAK, TNF-α-like weak inducer of apoptosis; PTX3, pentraxin 3; OSMR, oncostatin M receptor; EDA2R, ectodysplasin A2 receptor; NIK, NF-κB-inducing kinase; MDSCs, myeloid-derived suppressor cells; NLR, neutrocyte-lymphocyte ratio; LCN2, lipocalin 2; ATGL, adipose triglyceride lipase; HSL, hormone-sensitive lipase; UCP1, uncoupling protein-1; ZAG, zinc-α2-glycoprotein; CNDP1, carnosine dipeptidase 1; TIMP-1, tissue inhibitor of metalloproteinases-1.
Lipid metabolism in PC
In PC, lipolysis and adipocyte browning are critical phenomena associated with cachexia, serving as energy sources to fuel cancer cell proliferation. Lipolysis, the enzymatic cleavage of triglycerides into fatty acids and glycerol, is enhanced in PC cachexia, primarily catalyzed by enzymes such as adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL), with elevated expressions observed in this condition (28,29). Adipocyte browning, the conversion of white adipocytes to brown-like adipocytes, is characterized by an increase in uncoupling protein-1 (UCP1) activity, which shifts metabolism from energy storage to energy expenditure (30). UCP1, activated by adrenergic stimulation, forms part of a thermoporter complex that includes the mitochondrial calcium uniporter (MCU) and essential MCU regulator (EMRE), facilitating mitochondrial thermogenesis (31). Additionally, lipocalin 2 (LCN2), an adipocytokine secreted by PDAC-induced adipocytes, plays a dual role in enhancing thermogenesis via UCP1 and inducing fat and muscle degradation through ATGL and muscle RING-finger protein-1 (MURF-1) (32).
Zinc-α2-glycoprotein (ZAG), encoded by AZGP1, promotes lipolysis by upregulating UCP1 expression in brown adipose tissue (33) and is found in higher concentrations in PC patients with cachexia (34,35). Intriguingly, ZAG also impedes tumor progression in PDAC by inhibiting epithelial-mesenchymal transition through the transforming growth factor beta/extracellular signal-regulated kinase (TGF-β/ERK) signaling pathway (36).
Ceramides, essential components in lipid metabolism, modulate fatty acid absorption and utilization, prioritizing these processes over glucose metabolism, which can contribute to insulin resistance (37). In muscle cells, de novo synthesis of ceramides can trigger apoptosis (38). Furthermore, in PC, the ratio of C18:C24 ceramides serves as a distinguishing marker between cachectic patients and non-cachectic controls, highlighting its potential as a biomarker (39).
Parathyroid hormone-related protein (PTHrP) not only facilitates tumor growth and metastasis in PC but also significantly influences cachexia (40). Elevated levels of PTHrP are associated with weight loss and diminished handgrip strength, indicative of skeletal muscle depletion. The action of PTHrP shifts the energy balance toward a catabolic state, enhancing body fat oxidation and adipose tissue reduction, which are indicative of cachexia alongside skeletal muscle wasting (41).
Disruption of muscular homeostasis by KRAS-mutant PC
Protein synthesis and degradation are crucial determinants of skeletal muscle mass and function. The insulin-like growth factor 1 (IGF-1)/insulin signaling pathway plays a central role in regulating muscle size by enhancing protein synthesis and inhibiting protein breakdown. This pathway predominantly exerts its effects through the phosphoinositide 3 kinase (PI3K)/protein kinase B (Akt) signaling axis (42,43). Activation of Akt leads to the phosphorylation of forkhead box O (Foxo) transcription factors, notably inhibiting the translocation of Foxo3 to the nucleus. This inhibition prevents the transcription of ubiquitin ligases MAFbx/Atrogin-1 and MURF-1, which are integral to protein degradation (44). When the Akt/Foxo3 pathway is suppressed, these ubiquitin ligases become active, leading to the breakdown of key structural and regenerative proteins in skeletal muscle (Figure 2).

Additionally, the PI3K/Akt pathway modulates the expression of glucose transporter 4 (GLUT4), essential for insulin-mediated glucose uptake, serving both as a nutritional source for skeletal muscle cells and a systemic regulator of serum glucose levels (45). However, in PC, defects in the PI3K signaling pathway result in compromised glucose utilization in skeletal muscle and disruption of overall glucose metabolism (46).
The nuclear factor kappa B (NF-κB) signaling pathway also plays a significant role in muscle degradation, as NF-κB directly binds with the MURF-1 promoter, thereby promoting muscle catabolism (47). In PDAC patients, NF-κB activation is facilitated by ectodysplasin A2 receptor (EDA2R) through NF-κB-inducing kinase (NIK) activity, enhancing muscle atrophy via upregulated Atrogin-1 and MURF-1 expression. Transcriptional levels of EDA2R are notably higher in cachectic patients compared to non-cachectic individuals and non-cancer controls, and it is upregulated in response to tumor-induced oncostatin M (OSM) binding with its muscle-specific receptor, oncostatin M receptor (OSMR) (48). Targeting the OSMR/EDA2R/NIK signaling axis may thus offer therapeutic potential for mitigating muscle atrophy in cachexia.
Furthermore, in PC, the NF-κB pathway is hyperactivated due to systemic inflammation induced by cytokines from the TGF-β superfamily, which also activates the Janus kinase/signal transducer and activator of transcription 3 (JAK/STAT3) and SMAD family member 2 and 3 (SMAD2/3) pathways. These pathways collectively suppress myogenesis by downregulating myoblast determination protein (MyoD) (49,50), contributing to the muscle wasting observed in cachexia.
Ubiquitin proteasomal degradation in PC cachexia
Insulin receptor substrate 1 (IRS-1) and MG53
IRS-1 is a crucial element of the insulin receptor signaling pathway, further regulated by MG53, a ubiquitin E3 ligase. Elevated levels of circulating MG53 do not necessarily affect glucose handling or insulin signaling, yet there are conflicting reports regarding its role in glucose metabolism and myogenesis, particularly in PC mouse models (51,52). These discrepancies highlight the necessity for further research into MG53’s complex functions in PC cachexia.
Angiotensin II (Ang II)
Ang II acts as a significant early-stage regulator in cachexia, augmenting the proteolytic activity of ubiquitin-proteasome pathways, which leads to protein degradation in myotubes. This proteolysis can be mitigated by insulin-like growth factor (IGF) (53). Elevated plasma levels of Ang II are directly linked to skeletal muscle wasting and inversely associated with survival in PC patients (54).
Pancreatic adenocarcinoma upregulated factors (PAUFs)
PAUFs, secreted by PC cells, not only promotes tumor progression and metastasis (55) but also contributes to cachexia. Administration of PAUFs has been shown to induce body weight loss and muscle atrophy through mechanisms including the upregulation of Atrogin-1 via rapid deactivation of the IRS-1/Akt/Foxo3 signaling pathway (56).
Sirtuin1 (SIRT1)/NADPH oxidase 4 (NOX4) pathway
SIRT1, part of the silent information regulator 2 (SIR2) family, functions as an NAD+-dependent protein deacylase, regulating Foxo transcription factors (57). In PC, secreted factors reduce SIRT1 expression, activating Foxo and leading to increased levels of Atrogin-1 and MURF-1. Reduced SIRT1 activity also enhances NF-κB signaling, inducing oxidative stress through NOX4 (58). Inhibition of both SIRT1 and NOX4 has proven effective in reducing body weight loss and muscle atrophy, positioning the SIRT1/NOX4 pathway as a potential therapeutic target for cachexia in PC.
Toll-like receptor (TLR)/myeloid differentiation primary response gene 88 protein (MyD88)/XBP1 signaling
The MyD88 acts as a crucial adaptor for TLRs, excluding TLR3 and the type-1 interleukin receptor (IL-1R) family. In PDAC, MyD88 is associated with poor survival outcomes, primarily due to its role in systemic inflammation. Crucially, MyD88 is essential for PDAC cachexia progression, mediating the upregulation of Foxo1, Atrogin-1, and MURF-1 (59), suggesting its utility as a biomarker for this condition.
These interacting factors collectively promote skeletal muscle degradation over regeneration, contributing to the progressive decline of skeletal muscle in cachexia.
Autophagy-lysosome pathway
KRAS-mutant cells rely on nutrient-scavenging pathways, including macropinocytosis and autophagy-lysosome pathway, to release free biosynthetic precursors for cancer cell utilization. Consequently, autophagy emerges as the main catalyst of skeletal muscle proteolysis in PC under catabolic conditions. PDAC features an upregulation of autophagy, or enhanced macropinocytosis to offset autophagy blockade (60,61). In PC patients, systematic interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) induce muscular autophagy via inhibitory kappa B kinase alpha (IKKα)/NF-κB and AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) signaling, leading to skeletal muscle wasting (62,63). KRAS-mutant PC cells transport extracellular protein macropinocytosis to meet their metabolic demands (64), causing a one-way nutrient flux from skeletal muscle cells to PC cells.
PC-induced oxidative stress damages myotubes
NF-κB signaling
PC is often accompanied by chronic inflammation, which inflicts oxidative stress on skeletal muscle cells and results in cell damage directly. This oxidative damage is significantly influenced by the TGF-β superfamily. Key members, including myostatin, Atrogin-1 and growth differentiation factor 15 (GDF15), induce the production of pro-cachectic cytokines TNF-α, interleukin 1 beta (IL-1β), IL-6, monocyte chemoattractant protein 1/CC-motif chemokine ligand 2 (MCP-1/CCL2) and TNF-α-like weak inducer of apoptosis (TWEAK) (65-67). These cytokines activate the NF-κB signaling pathway, which contributes to skeletal muscle wasting by inducing oxidative stress, which is a known facilitator of muscle atrophy (68). While MCP-1/CCL2 levels upon diagnosis correlate with future skeletal muscle wasting (67), the reliability of these cytokines as biomarkers of cancer cachexia is yet to be established, warranting further investigation (Figure 2).
Activin A, another TGF-β superfamily member expressed by PDAC cells, promotes both tumor growth and cachexia, with variations based on sex (69). PDAC cells release soluble factors, triggering Activin A secretion from both tumor tissue and distant organs, correlating with increased cachexia severity and myotube atrophy. Notably, cachexia presents less severely in females, potentially due to estradiol’s influence (65). Targeting Activin A signaling appears to inhibit tumor progression and muscle wasting, thus extending survival (70,71), indicating its significant prognostic and therapeutic potential.
The pentraxin 3 (PTX3) gene, with NF-κB binding sites (72,73), encodes a humoral pattern recognition protein elevated in PC patients (72). Serum PTX3 levels have been identified as a risk factor for skeletal muscle and correlate with inflammatory markers as well as disease severity (74), suggesting its utility as a biomarker for PC and related cachexia.
Biomarkers for assessing skeletal muscle atrophy in KRAS-mutant PC
Myofiber components
Detection of altered levels of skeletal muscle cell components in the serum, particularly during muscle wasting, is a key diagnostic indicator in PC. β-dystroglycan, a central component of the dystroglycan complex, plays a pivotal role in skeletal muscle cells by linking the intracellular actin cytoskeleton to the extracellular matrix (75). In PC patients, β-dystroglycan undergoes aberrant glycosylation and shows significant upregulation. These changes have led to its adoption as a marker for cachexia in clinical settings (76,77).
Carnosine, predominantly located in the muscle tissue, is metabolized by carnosine dipeptidase 1 (CNDP1). Intriguingly, circulating levels of CNDP1 show a positive correlation with survival rates, BMI and fat mass in PC patients. Conversely, these levels inversely associate with percentage of weight loss and other cachexia-related factors (78).
Extracellular matrix
Tissue inhibitor of metalloproteinases-1 (TIMP-1) have emerged as a crucial regulator of matrix metalloproteinase (MMP) activity. In PC, TIMP-1, along with intercellular adhesion molecule 1 (ICAM1), has been identified as a biomarker superior to carbohydrate antigen 19-9 (CA19-9) in global quantitative proteomics profiling (79). Additionally, TIMP-1 upregulation positively correlates with weight loss and serves as a prognostic indicator in cachectic PDAC patients without jaundice (80).
Non-coding RNAs (ncRNAs)
ncRNAs, particularly microRNAs (miRNAs), are emerging cachexia mediators in PC cachexia. MiR-21, secreted by micro-vesicles, activates the toll-like receptor 7/c-Jun N-terminal kinase (TLR7/JNK) pathway, inducing myoblast apoptosis (81). Serum miR-155 levels correlate with the severity of PC cachexia, influencing TNF-α signaling and regulatory T cell (Treg) function, as well as TLR signaling via suppressor of cytokine signalling 1 (SOCS1), forkhead box P3 (Foxp3) and TGF-β-activated kinase 1 binding protein 2 (TAB2) (82-85). Both miR-21 and miR-155 are overexpressed in intraductal papillary mucinous neoplasms (IPMNs) lesions, precursors of PC, suggesting their potential as predictive biomarkers for both PC and associated cachexia (86).
MiR-let-7b-5p, a PC-derived exosomal miRNA, targets RNF20, an E3 ubiquitin ligase. This interaction alleviates insulin resistance in myotube cells by deactivating the STAT3/Foxo1/GLUT4 axis (87). In PC, ZIP4 activates the cyclic adenosine monophosphate (cAMP) response element binding protein (CREB)-miR-373-PH domain and leucine-rich repeat protein phosphatase 2 (PHLPP2) feed-forward loop, where the suppression of PHLPP2 promotes both tumor growth and cachexia by dephosphorylating Akt and inhibiting downstream CyclinD1 and STAT5-TGF-β signaling (88). CircANAPC7, a long non-coding circular RNA, functions as a miR-373 sponge, counteracting the suppression of PHLPP2, and inhibiting skeletal muscle wasting (89). In the adipose tissue of PC patients, ncRNAs modulates lipid metabolism and thermogenesis. MiRNAs such as miR-30b/c, miR-494-3p, miR-9, miR-338-3p, miR-106b, miR-93, and miR-27b are implicated in the regulation of UCP1 and other thermogenic proteins (90-94).
Immune cell remodeling in PC-induced sarcopenia and cachexia
The orchestration of immune responses plays a pivotal role in skeletal muscle regulation, where a delicate equilibrium is maintained between the removal of damaged muscle fibers and the promotion of myogenesis. This balance is disrupted in the immunosuppressive milieu of PC, dominated by an abundance of M2-like macrophages, myeloid-derived suppressor cells (MDSCs), and Tregs, overshadowing the roles of M1-like macrophages and effector T cells (95,96) (Figure 3).

Macrophages are central to the repair and remodeling of skeletal muscle. Initially, circulating monocytes are recruited to the site of injury, differentiating into M1-like macrophages that facilitate the clearance of necrotic tissue and support satellite cell proliferation (97). Subsequently, a phenotypic transition to M2-like macrophages occurs, favoring myogenesis (98). In the context of PC, however, M2-like macrophages synergize with tumor cells, promoting muscle wasting via STAT3 signaling (99) and crosstalk. PC cells overexpress CCL2, which upregulates CC-motif chemokine ligand 5 (CCL5) secreted by M2 macrophages, stimulating PC cells to release TWEAK via CCL5/TNF receptor associated factor 6 (TRAF6)/NF-κB signaling, leading to skeletal muscle wasting through MURF-1 activation (100).
Furthermore, Kupffer cells, liver-resident macrophages, exhibit a correlation with both nutritional decline and tumor proliferation in PC (101).
Neutrophils, as initial responders to muscle injury, primarily focus on clearing debris. However, their prolonged presence can hinder muscle regeneration (102). PC cachexia is characterized by increased neutrophil counts and the upregulation of neutrophil-derived proteases, such as Cathepsin B (54), with neutrophils also being a significant source of LCN2, a mediator of both metabolic regulation and anorexia (103,104). Moreover, the presence of Ly6G+ neutrophils and granulocytic MDSCs (gMDSCs) has been linked to muscle wasting in PC (105), with the neutrophil/lymphocyte ratio serving as a biomarker for cachexia-related inflammation (54).
T lymphocytes, particularly CD8+ cells, exert control over muscle mass independently and through modulation of macrophage function (106-108). Cachexia in early-stage PDAC is associated with diminished levels of tumor-infiltrating CD8+ T cells (109), where the activation of TLR7 on CD8+ T cells has been shown to counteract cachexia and limit tumor growth (110,111), underscoring a crucial inverse relationship between CD8+ T cell presence and cachexia. Conversely, the roles of CD4+ T helper cells and Tregs appear unchanged in cachexia, though a decrease in liver IL-4 mRNA has been observed, indicating a potential area for further exploration regarding CD4+ T cells’ involvement in cachexia (98).
Current therapeutic interventions for PC-associated cachexia
Current therapeutic strategies against cachexia focus on restoring the balance between skeletal muscle degeneration and regeneration. Efforts to promote muscle regeneration include countering anorexia, nutritional supplementation, and encouraging physical activity, while strategies to discourage muscle degeneration involve suppressing inflammation and other catabolic signaling pathways (Table 3).
Table 3
| Pharmaceutical therapy | Therapeutical target or biochemical feature | Compound | Clinical trial No. | Phase and design | Population | N | Primary outcomes | Cachexia/sarcopenia | Study start and completion dates | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Prophagic therapy | GHS-R | Anamorelin | NCT04844970 | II, randomized, double blind, placebo-controlled | US, unresectable or metastatic PDAC | 100 | Weight change | Cachexia | April 01, 2023 to NP | Recruiting |
| Macimorelin | NCT01614990 | II, randomized, triple blind, placebo-controlled | US, incurable solid tumor | 15 | Change in body weight, IGF-1 plasma levels and QoL score | Cachexia | May 2012 to December 2012 | Published (112) | ||
| GDF15 | Ponsegromab | NCT05546476 | II, randomized, double blind, placebo-controlled | US, NSCLC, pancreatic, colorectal cancer | 187 | Weight change | Cachexia | November 21, 2022 to March 13, 2024 | Active, not recruiting | |
| Progestogens | Progesterone | Megestrol acetate | NCT00637728 | III, randomized, double blind, placebo-controlled | US, stage II, III, or IV lung or pancreatic cancer | 5 | Caloric intake | Cachexia | June 2006 to September 2006 | Completed (not published) |
| Androgen | Decanoate | NCT03263520 | Not applicable, randomized, double-blind, vs. dexamethasone | Brazil, palliative high gastro-intestinal, hepatobiliary and pancreatic cancer | 60 | BMI, body weight, body composition, QoL score | Cachexia, sarcopenia | February 2016 to October 31, 2017 | Completed (not published) | |
| Pro-myogenesis | TNF-α | Infliximab | NCT00060502 | II, randomized, double blind, placebo-controlled | US, newly diagnosed pancreatic cancer | 73 | Change in lean body mass | Sarcopenia | April 2003 to February 2006 | Completed (113) |
| Thalidomide | NCT06017284 | III, randomized, double blind, placebo-controlled | China, metastatic PDAC | 100 | Rate of nausea/vomiting | Cachexia | November 01, 2023 to NP | Recruiting | ||
| IL-1α | Xilonix | NCT03207724 | I, open-label, single group assignment | US, advanced or locally advanced pancreatic cancer | 16 | Maximum tolerated dose of Xilonix and onivyde, 5-fluorouracil/folinic acid in combination with Xilonix | Neither | October 16, 2017 to October 27, 2020 | Completed (114) | |
| Activin type 2 receptors | Bimagrumab | NCT01433263 | II, randomized, double blind, placebo-controlled | Lithuania, Romania, Switzerland, UK, US, stage IV NSCLC or stage III/IV pancreatic adenocarcinoma | 57 | Thigh muscle volume change | Sarcopenia | August 2011 to April 2014 | Completed (not published) | |
| Myostatin | LY2495655 | NCT01505530 | II, randomized, triple blind, placebo-controlled | US, unresectable or metastatic pancreas cancer | 125 | Overall survival | Neither | December 2011 to January 2016 | Completed (not published) | |
| Anti-inflammation | Nutrients | EPA and DHA | NCT02681601 | II, randomized, open-label vs. standard dietary intervention | US, unresectable PDAC | 2 | Body weight and body composition | Cachexia, sarcopenia | July 19, 2016 to October 01, 2021 | Terminated |
| NSAIDs | Ibuprofen | Unknown | Unknown | Pancreatic cancer | 16 | Resting energy expenditure, serum CRP level | Cachexia | Unknown | Completed (115) | |
| Celecoxib | IRCT201407222027N4 | III, randomized, triple blind, placebo-controlled | Iran, esophageal, gastric, colorectal and pancreatic cancer | 90 | Quality of life, body weight | Cachexia | October 2015 to NP | Completed (116) | ||
| NSAIDs | Ketorolac acid | NCT05336266 | Early I, open-label, single group assignment | US, advanced and refractory PDAC | 20 | Patient compliance | Neither | July 01, 2022 to NP | Recruiting | |
| Corticosteroids | Prednisolone and dexamethasone | – | – | – | – | – | – | – | – | – |
GHS-R, growth hormone secretagogue receptor; PDAC, pancreatic ductal adenocarcinoma; NP, not published; IGF-1, insulin-like growth factor 1; QoL, quality of life; GDF15, growth differentiation factor 15; NSCLC, non-small cell lung cancer; BMI, body mass index; TNF-α, tumor necrosis factor alpha; IL-1α, interleukin 1 alpha; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; NSAIDs, non-steroidal anti-inflammatory drugs; CRP, C-reactive protein.
Prophagic therapy
In PC cachexia, the central nervous system is an integral and complex regulator of appetite. Inflammation in the PC milieu suppresses prophagic responses and enhances anorexigenic responses in the hypothalamus by endocrine hormones leptin and ghrelin (117). Growth hormone secretagogue receptor (GHS-R) functions as ghrelin’s receptor and controls the release of growth hormone as well, the latter an upstream regulator of IGF-1 in the muscle tissue. GHS-R delivers outstanding performance as a therapeutic target. Its antagonists, anamorelin and macimorelin are rising stars in the field of cachexia therapy. Anamorelin for PC associated cachexia treatment made its debut with the ONO-7643 trial and landed approval in Japan in 2020 (118,119). It significantly improves body weight, lean body mass and appetite (118), whereas responsiveness is associated with higher total protein, albumin, transferrin and prognostic nutritional index, and lower neutrophil/lymphocyte and C-reactive protein (CRP)/albumin ratios (120). Anamorelin is still undergoing phase II clinical trials in PC patients in the US (NCT04844970). Macimorelin awaits further investigation based on its pilot trial (NCT01614990) (112). In the pilot trial, continual daily oral administration of macimorelin lasted one week. Although no statistic difference in body mass, physical function endpoints, appetite, food intake or energy expenditure was observed, body weight and quality of life exhibit numerical improvement (112). Anorexia is also induced by GDF15/GDNF family receptor alpha-like (GFRAL) signaling (121). GFRAL’s ligand GDF15 is a potential therapeutical target for PC-associated cachexia. Ponsegromab (PF-06946860), a humanized monoclonal antibody (mAb) against GDF15, is currently under phase II trial (NCT05546476).
Progestogens
Megestrol acetate is a progesterone derivative earliest used for acquired immunodeficiency syndrome (AIDS)-associated cachexia treatment as an appetite stimulant (122). Its anti-cachectic usage has extended to cancer-associated cachexia, effectively improving appetite and body weight in PC patients (NCT00637728) (123,124). Nandrolone decanoate is a minor endogenous androgen that managed to improve body weight, lean body mass and functionality in human immunodeficiency virus (HIV)-afflicted cachexia patients (125,126). A trial has been conducted on nandrolone decanoate’s efficacy for treating malnutrition in cancer patients, which included cachectic PC patients (NCT03263520), though no results have been published yet.
Inflammation suppression
In PC-associated cachexia, the integrated JAK/STAT3 and SMAD2/3 signaling aforementioned pathway is activated upon cytokine, activin and myostatin stimulation, which downregulates the expression of MyoD, a pro-myogenesis molecule. Cytokines, especially TNF-α and interleukin 1 alpha (IL-1α) (82), as well as activin and myostatin, are potential therapeutic targets in cachexia treatment. Infliximab and thalidomide are TNF-α suppressants. Infliximab is a chimeric IgG1-κ mAb against TNF-α, while thalidomide is an inhibitor of TNF-α synthesis. However, neither drug resulted in significant improvement in cachexia in PC patients (113,127-129).
Xilonix or bermekimab (MABp1) is a mAb specific to human IL-1α. It is currently under phase I trial (NCT03207724).
Bimagrumab (BYM338) is a human mAb against activin type 2 receptors. LY2495655 is a humanized IgG1 mAb against myostatin. Both studies have completed phase II trials on PC patients but neither exhibited promising results (130) (NCT01433263, NCT01505530).
Anti-inflammatory nutrients
Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are ω3 or n-3 polyunsaturated fatty acids (PUFAs) found in cold-water fish. In PC patients, EPA managed to stabilize body weight as well as reduce CRP, IL-6 and cortisol-to-insulin ratio, while improving serum insulin, creating a pro-myogenesis profile (131-133). However, there were controversial results showing EPA failed to outperform placebo in weight stabilization (134,135). EPA plays an anti-cachectic role by inhibiting proteolysis, enhancing protein synthesis and aiding chemotherapy, and is most effective when provided in early stages of cachexia development (136,137).
Non-steroidal anti-inflammatory drug (NSAIDs) and corticosteroids
Ibuprofen and celecoxib are selective COX2 inhibitors. Ibuprofen was shown to reduce resting energy expenditure as well as CRP levels, and celecoxib resulted in weight gain when combined with megestrol acetate in PC patients with cachexia (115,116,138). Ketorolac acid is a COX enzyme inhibitor, also known as a NSAID. In cancer-bearing mice with cachexia, it has shown to prolong survival and ameliorate weight, adipose and muscle loss in a T-cell-dependent manner (139). It is still recruiting candidates for an early phase I trial on advanced and refractory PDAC patients (NCT05336266). Short-term usage of corticosteroids in treating cancer-associated cachexia already acquired moderate strength recommendation (140).
Anti-inflammatory diet
The Mediterranean diet is a balanced, pro-health structure based on plant-origin foods, delivering a broad spectrum of nutrients and fibers essential to overall health. It also comprises moderate seafood, which is abundant in ω3 or n-3 PUFAs. The food pyramid of the Mediterranean diet includes fruit, vegetables and cereal as its bottom layer. Virgin olive oil is rich in monounsaturated oleic acids and antioxidant compounds, and it serves as the major culinary fat. Other natural sources of lipids include olives, nuts and seeds. Fish, shellfish, white meat and eggs are moderately consumed as the main source of animal protein instead of red meats. In addition to food consumption, culinary and physical activity in a sense of socialization as well as adequate rest is also an essential part of the Mediterranean lifestyle, contributing to an overall health and well-being (141). A study on stage III–IV colorectal cancer patients with cachexia delivered promising results. The Mediterranean diet increased weight, lean body mass, muscle strength, the global health status score and physical performance score while lowering TNF-α, high-sensitive-C-reactive protein (hs-CRP) and IL-6 serum levels (IRCT20211027052884N1) (142). These results may shed light on future applications in PC.
Physical activity
Exercise, according to the 2020 American Society of Clinical Oncology (ASCO) guideline, awaits further evaluation to be approved for effective cancer-associated cachexia treatment (140). It is currently under investigation as a part of multimodal trials on PC patients with cachexia (NCT05420259 and NCT04907864).
These therapeutic interventions represent a multifaceted approach to tackling the complex issue of cachexia in PC, with a mix of pharmacological, nutritional, and lifestyle modifications aimed at improving patient outcome.
Conclusions
Comprehensive evidence updates the intricate crosstalk between KRAS-mutant PC and skeletal muscle atrophy, delineating the pathophysiological context essential for grasping sarcopenia and associated disorders. Thorough analysis enriches our knowledge of the complex causes of PC cachexia and sarcopenia and promotes the development of targeted therapeutic strategies.
Acknowledgments
Funding: This work was supported by grants from
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-282/rc
Peer Review File: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-282/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-24-282/coif). W.L. serves as an unpaid editorial board member of HepatoBiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr 2008;27:793-9. [Crossref] [PubMed]
- Mitsunaga S, Kasamatsu E, Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer 2020;28:5271-9. [Crossref] [PubMed]
- Sánchez-Lara K, Ugalde-Morales E, Motola-Kuba D, et al. Gastrointestinal symptoms and weight loss in cancer patients receiving chemotherapy. Br J Nutr 2013;109:894-7. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Zhou T, Wang B, Liu H, et al. Development and validation of a clinically applicable score to classify cachexia stages in advanced cancer patients. J Cachexia Sarcopenia Muscle 2018;9:306-14. [Crossref] [PubMed]
- Arends J, Strasser F, Gonella S, et al. Cancer cachexia in adult patients: ESMO Clinical Practice Guidelines ESMO Open 2021;6:100092. [Crossref] [PubMed]
- Cederholm T, Jensen GL, Correia MITD, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. Clin Nutr 2019;38:1-9. [Crossref] [PubMed]
- Hendifar AE, Chang JI, Huang BZ, et al. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J Gastrointest Oncol 2018;9:17-23. [Crossref] [PubMed]
- Bachmann J, Ketterer K, Marsch C, et al. Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC Cancer 2009;9:255. [Crossref] [PubMed]
- van Vledder MG, Levolger S, Ayez N, et al. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg 2012;99:550-7. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]
- Tan BH, Birdsell LA, Martin L, et al. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res 2009;15:6973-9. [Crossref] [PubMed]
- Parsons HA, Baracos VE, Dhillon N, et al. Body composition, symptoms, and survival in advanced cancer patients referred to a phase I service. PLoS One 2012;7:e29330. [Crossref] [PubMed]
- Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629-35. [Crossref] [PubMed]
- Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011;47:1928-37. [Crossref] [PubMed]
- Wang Z, Lai ST, Xie L, et al. Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract 2014;106:19-26. [Crossref] [PubMed]
- Hu CM, Tien SC, Hsieh PK, et al. High Glucose Triggers Nucleotide Imbalance through O-GlcNAcylation of Key Enzymes and Induces KRAS Mutation in Pancreatic Cells. Cell Metab 2019;29:1334-1349.e10. [Crossref] [PubMed]
- Liu YH, Hu CM, Hsu YS, et al. Interplays of glucose metabolism and KRAS mutation in pancreatic ductal adenocarcinoma. Cell Death Dis 2022;13:817. [Crossref] [PubMed]
- Herner A, Sauliunaite D, Michalski CW, et al. Glutamate increases pancreatic cancer cell invasion and migration via AMPA receptor activation and Kras-MAPK signaling. Int J Cancer 2011;129:2349-59. [Crossref] [PubMed]
- Wang F, Liu H, Hu L, et al. The Warburg effect in human pancreatic cancer cells triggers cachexia in athymic mice carrying the cancer cells. BMC Cancer 2018;18:360. [Crossref] [PubMed]
- Neyroud D, Laitano O, Dasgupta A, et al. Blocking muscle wasting via deletion of the muscle-specific E3 ligase MuRF1 impedes pancreatic tumor growth. Commun Biol 2023;6:519. [Crossref] [PubMed]
- Yin M, Zhang H, Liu Q, et al. Determination of skeletal muscle mass by aspartate aminotransferase / alanine aminotransferase ratio, insulin and FSH in Chinese women with sarcopenia. BMC Geriatr 2022;22:893. [Crossref] [PubMed]
- Ren LL, Mao T, Meng P, et al. Glutamine addiction and therapeutic strategies in pancreatic cancer. World J Gastrointest Oncol 2023;15:1852-63. [Crossref] [PubMed]
- Krishnamachary B, Sivakumar I, Mironchik Y, et al. Downregulation of glutamine transporter stably and significantly attenuates weight loss by a cachexia-inducing pancreatic cancer xenograft. Cancer Res 2022;82:abstr 6305.
- Raho S, Capobianco L, Malivindi R, et al. KRAS-regulated glutamine metabolism requires UCP2-mediated aspartate transport to support pancreatic cancer growth. Nat Metab 2020;2:1373-81. [Crossref] [PubMed]
- Rozeveld CN, Johnson KM, Zhang L, et al. KRAS Controls Pancreatic Cancer Cell Lipid Metabolism and Invasive Potential through the Lipase HSL. Cancer Res 2020;80:4932-45. [Crossref] [PubMed]
- Lee JS, Oh SJ, Choi HJ, et al. ATP Production Relies on Fatty Acid Oxidation Rather than Glycolysis in Pancreatic Ductal Adenocarcinoma. Cancers (Basel) 2020;12:2477. [Crossref] [PubMed]
- Agustsson T, Rydén M, Hoffstedt J, et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res 2007;67:5531-7. [Crossref] [PubMed]
- Das SK, Eder S, Schauer S, et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 2011;333:233-8. [Crossref] [PubMed]
- Petruzzelli M, Schweiger M, Schreiber R, et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab 2014;20:433-47. [Crossref] [PubMed]
- Xue K, Wu D, Wang Y, et al. The mitochondrial calcium uniporter engages UCP1 to form a thermoporter that promotes thermogenesis. Cell Metab 2022;34:1325-1341.e6. [Crossref] [PubMed]
- Lemecha M, Chalise JP, Takamuku Y, et al. Lcn2 mediates adipocyte-muscle-tumor communication and hypothermia in pancreatic cancer cachexia. Mol Metab 2022;66:101612. [Crossref] [PubMed]
- Russell ST, Zimmerman TP, Domin BA, et al. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochim Biophys Acta 2004;1636:59-68. [Crossref] [PubMed]
- Felix K, Fakelman F, Hartmann D, et al. Identification of serum proteins involved in pancreatic cancer cachexia. Life Sci 2011;88:218-25. [Crossref] [PubMed]
- Bing C. Lipid mobilization in cachexia: mechanisms and mediators. Curr Opin Support Palliat Care 2011;5:356-60. [Crossref] [PubMed]
- Kong B, Michalski CW, Hong X, et al. AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-β-mediated ERK signaling. Oncogene 2010;29:5146-58. [Crossref] [PubMed]
- Summers SA, Chaurasia B, Holland WL. Metabolic Messengers: ceramides. Nat Metab 2019;1:1051-8. [Crossref] [PubMed]
- Turpin SM, Lancaster GI, Darby I, et al. Apoptosis in skeletal muscle myotubes is induced by ceramides and is positively related to insulin resistance. Am J Physiol Endocrinol Metab 2006;291:E1341-50. [Crossref] [PubMed]
- Chakedis JM, Dillhoff ME, Schmidt CR, et al. Identification of circulating plasma ceramides as a potential sexually dimorphic biomarker of pancreatic cancer-induced cachexia. JCSM Rapid Commun 2022;5:254-65. [Crossref] [PubMed]
- Pitarresi JR. Altered calcium signaling in cancer: PTHrP-KRAS collateral amplification governs pancreatic cancer metastasis, cachexia, and immunosuppression. Cancer Res 2023;83:abstr NG12.
- Iresjö BM, Kir S, Lundholm K. Parathyroid hormone related protein (PTHrP) in patients with pancreatic carcinoma and overt signs of disease progression and host tissue wasting. Transl Oncol 2023;36:101752. [Crossref] [PubMed]
- Fanzani A, Conraads VM, Penna F, et al. Molecular and cellular mechanisms of skeletal muscle atrophy: an update. J Cachexia Sarcopenia Muscle 2012;3:163-79. [Crossref] [PubMed]
- Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 2001;3:1014-9. [Crossref] [PubMed]
- Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 2004;117:399-412. [Crossref] [PubMed]
- Mann G, Riddell MC, Adegoke OAJ. Effects of Acute Muscle Contraction on the Key Molecules in Insulin and Akt Signaling in Skeletal Muscle in Health and in Insulin Resistant States. Diabetology 2022;3:423-46.
- Isaksson B, Strömmer L, Friess H, et al. Impaired insulin action on phosphatidylinositol 3-kinase activity and glucose transport in skeletal muscle of pancreatic cancer patients. Pancreas 2003;26:173-7. [Crossref] [PubMed]
- Wu CL, Cornwell EW, Jackman RW, et al. NF-κB but not FoxO sites in the MuRF1 promoter are required for transcriptional activation in disuse muscle atrophy. Am J Physiol Cell Physiol 2014;306:C762-7. [Crossref] [PubMed]
- Bilgic SN, Domaniku A, Toledo B, et al. EDA2R-NIK signalling promotes muscle atrophy linked to cancer cachexia. Nature 2023;617:827-34. [Crossref] [PubMed]
- Hall DT, Ma JF, Marco SD, et al. Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging (Albany NY) 2011;3:702-15. [Crossref] [PubMed]
- Yang Y, Xu Y, Li W, et al. STAT3 induces muscle stem cell differentiation by interaction with myoD. Cytokine 2009;46:137-41. [Crossref] [PubMed]
- Bian Z, Wang Q, Zhou X, et al. Sustained elevation of MG53 in the bloodstream increases tissue regenerative capacity without compromising metabolic function. Nat Commun 2019;10:4659. [Crossref] [PubMed]
- Yi JS, Park JS, Ham YM, et al. MG53-induced IRS-1 ubiquitination negatively regulates skeletal myogenesis and insulin signalling. Nat Commun 2013;4:2354. [Crossref] [PubMed]
- Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin-proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer 2005;93:425-34. [Crossref] [PubMed]
- Penafuerte CA, Gagnon B, Sirois J, et al. Identification of neutrophil-derived proteases and angiotensin II as biomarkers of cancer cachexia. Br J Cancer 2016;114:680-7. [Crossref] [PubMed]
- Kim SJ, Chang S, Lee Y, et al. A PAUF-neutralizing antibody targets both carcinoma and endothelial cells to impede pancreatic tumor progression and metastasis. Biochem Biophys Res Commun 2014;454:144-50. [Crossref] [PubMed]
- Yoo W, Choi H, Son YH, et al. Pancreatic cancer induces muscle wasting by promoting the release of pancreatic adenocarcinoma upregulated factor. Exp Mol Med 2021;53:432-45. [Crossref] [PubMed]
- Yang Y, Hou H, Haller EM, et al. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J 2005;24:1021-32. [Crossref] [PubMed]
- Dasgupta A, Shukla SK, Vernucci E, et al. SIRT1-NOX4 signaling axis regulates cancer cachexia. J Exp Med 2020;217:e20190745. [Crossref] [PubMed]
- Zhu X, Burfeind KG, Michaelis KA, et al. MyD88 signalling is critical in the development of pancreatic cancer cachexia. J Cachexia Sarcopenia Muscle 2019;10:378-90. [Crossref] [PubMed]
- Regel I, Mayerle J. Nutrient Scavenging From Muscle Cells: A Survival Strategy of Pancreatic Cancer Cells Ends in Cachexia. Gastroenterology 2022;163:1161-3. [Crossref] [PubMed]
- Su H, Yang F, Fu R, et al. Cancer cells escape autophagy inhibition via NRF2-induced macropinocytosis. Cancer Cell 2021;39:678-693.e11. [Crossref] [PubMed]
- Pettersen K, Andersen S, Degen S, et al. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci Rep 2017;7:2046. [Crossref] [PubMed]
- Piffoux M, Eriau E, Cassier PA. Autophagy as a therapeutic target in pancreatic cancer. Br J Cancer 2021;124:333-44. [Crossref] [PubMed]
- Commisso C, Davidson SM, Soydaner-Azeloglu RG, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 2013;497:633-7. [Crossref] [PubMed]
- Zhong X, Narasimhan A, Silverman LM, et al. Sex specificity of pancreatic cancer cachexia phenotypes, mechanisms, and treatment in mice and humans: role of Activin. J Cachexia Sarcopenia Muscle 2022;13:2146-61. [Crossref] [PubMed]
- Antonio-Herrera L, Bergthaler A. Molecular basis for muscle loss that causes cachexia. Nature 2023;617:684-5. [Crossref] [PubMed]
- Babic A, Rosenthal MH, Sundaresan TK, et al. Adipose tissue and skeletal muscle wasting precede clinical diagnosis of pancreatic cancer. Nat Commun 2023;14:4317. [Crossref] [PubMed]
- Williams G, Brown T, Becker L, et al. Cytokine-induced expression of nitric oxide synthase in C2C12 skeletal muscle myocytes. Am J Physiol 1994;267:R1020-5. [Crossref] [PubMed]
- Zhong X, Narasimhan A, Young AR, et al. Sex differences in pancreatic cancer cachexia manifestations and mechanisms in mice and humans: Role of activin. Cancer Res 2021;81:abstr 2657.
- Zhong X, Pons M, Poirier C, et al. The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy. J Cachexia Sarcopenia Muscle 2019;10:1083-101. [Crossref] [PubMed]
- Togashi Y, Kogita A, Sakamoto H, et al. Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Cancer Lett 2015;356:819-27. [Crossref] [PubMed]
- Garlanda C, Bottazzi B, Magrini E, et al. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol Rev 2018;98:623-39. [Crossref] [PubMed]
- Li D, Hao Z, Nan Y, et al. Role of long pentraxin PTX3 in cancer. Clin Exp Med 2023;23:4401-11. [Crossref] [PubMed]
- Sato K, Hikita H, Shigekawa M, et al. Pentraxin 3 is an adipose tissue-related serum marker for pancreatic cancer cachexia predicting subsequent muscle mass and visceral fat loss. Cancer Sci 2022;113:4311-26. [Crossref] [PubMed]
- Werle MJ. Neuromuscular Junction (NMJ): Postsynaptic Basal Lamina. In: Squire LR. editor. Encyclopedia of Neuroscience. Oxford: Academic Press; 2009:595-600.
- Stephens NA, Skipworth RJ, Gallagher IJ, et al. Evaluating potential biomarkers of cachexia and survival in skeletal muscle of upper gastrointestinal cancer patients. J Cachexia Sarcopenia Muscle 2015;6:53-61. [Crossref] [PubMed]
- Matsumura K, Zhong D, Saito F, et al. Proteolysis of beta-dystroglycan in muscular diseases. Neuromuscul Disord 2005;15:336-41. [Crossref] [PubMed]
- Arner P, Henjes F, Schwenk JM, et al. Circulating carnosine dipeptidase 1 associates with weight loss and poor prognosis in gastrointestinal cancer. PLoS One 2015;10:e0123566. [Crossref] [PubMed]
- Pan S, Chen R, Crispin DA, et al. Protein alterations associated with pancreatic cancer and chronic pancreatitis found in human plasma using global quantitative proteomics profiling. J Proteome Res 2011;10:2359-76. [Crossref] [PubMed]
- Prokopchuk O, Grünwald B, Nitsche U, et al. Elevated systemic levels of the matrix metalloproteinase inhibitor TIMP-1 correlate with clinical markers of cachexia in patients with chronic pancreatitis and pancreatic cancer. BMC Cancer 2018;18:128. [Crossref] [PubMed]
- He WA, Calore F, Londhe P, et al. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci U S A 2014;111:4525-9. [Crossref] [PubMed]
- Yehia R, Schaalan M, Abdallah DM, et al. Impact of TNF-α Gene Polymorphisms on Pancreatic and Non-Small Cell Lung Cancer-Induced Cachexia in Adult Egyptian Patients: A Focus on Pathogenic Trajectories. Front Oncol 2021;11:783231. [Crossref] [PubMed]
- Wang P, Hou J, Lin L, et al. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J Immunol 2010;185:6226-33. [Crossref] [PubMed]
- Gerriets VA, Kishton RJ, Johnson MO, et al. Foxp3 and Toll-like receptor signaling balance T(reg) cell anabolic metabolism for suppression. Nat Immunol 2016;17:1459-66. [Crossref] [PubMed]
- Takaesu G, Kishida S, Hiyama A, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell 2000;5:649-58. [Crossref] [PubMed]
- Habbe N, Koorstra JB, Mendell JT, et al. MicroRNA miR-155 is a biomarker of early pancreatic neoplasia. Cancer Biol Ther 2009;8:340-6. [Crossref] [PubMed]
- Wang L, Li X, Wu J, et al. Pancreatic Cancer-Derived Exosomal miR-Let-7b-5p Stimulates Insulin Resistance in Skeletal Muscle Cells Through RNF20/STAT3/FOXO1 Axis Regulation. Diabetes Metab Syndr Obes 2023;16:3133-45. [Crossref] [PubMed]
- Zhang Y, Yang J, Cui X, et al. A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth. EMBO Mol Med 2013;5:1322-34. [Crossref] [PubMed]
- Shi X, Yang J, Liu M, et al. Circular RNA ANAPC7 Inhibits Tumor Growth and Muscle Wasting via PHLPP2-AKT-TGF-β Signaling Axis in Pancreatic Cancer. Gastroenterology 2022;162:2004-2017.e2. [Crossref] [PubMed]
- Hu F, Wang M, Xiao T, et al. miR-30 promotes thermogenesis and the development of beige fat by targeting RIP140. Diabetes 2015;64:2056-68. [Crossref] [PubMed]
- Wu Y, Zuo J, Zhang Y, et al. Identification of miR-106b-93 as a negative regulator of brown adipocyte differentiation. Biochem Biophys Res Commun 2013;438:575-80. [Crossref] [PubMed]
- Zheng Z, Liu X, Zhao Q, et al. Regulation of UCP1 in the Browning of Epididymal Adipose Tissue by β3-Adrenergic Agonist: A Role for MicroRNAs. Int J Endocrinol 2014;2014:530636. [Crossref] [PubMed]
- Lemecha M, Morino K, Imamura T, et al. MiR-494-3p regulates mitochondrial biogenesis and thermogenesis through PGC1-α signalling in beige adipocytes. Sci Rep 2018;8:15096. [Crossref] [PubMed]
- Wang X, Chen S, Lv D, et al. Liraglutide suppresses obesity and promotes browning of white fat via miR-27b in vivo and in vitro. J Int Med Res 2021;49:3000605211055059. [Crossref] [PubMed]
- Murakami T, Hiroshima Y, Matsuyama R, et al. Role of the tumor microenvironment in pancreatic cancer. Ann Gastroenterol Surg 2019;3:130-7. [Crossref] [PubMed]
- Torphy RJ, Schulick RD, Zhu Y. Understanding the immune landscape and tumor microenvironment of pancreatic cancer to improve immunotherapy. Mol Carcinog 2020;59:775-82. [Crossref] [PubMed]
- Ceafalan LC, Fertig TE, Popescu AC, et al. Skeletal muscle regeneration involves macrophage-myoblast bonding. Cell Adh Migr 2018;12:228-35. [Crossref] [PubMed]
- Hammers DW, Rybalko V, Merscham-Banda M, et al. Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia-reperfusion. J Appl Physiol (1985) 2015;118:1067-74. [Crossref] [PubMed]
- Shukla SK, Markov SD, Attri KS, et al. Macrophages potentiate STAT3 signaling in skeletal muscles and regulate pancreatic cancer cachexia. Cancer Lett 2020;484:29-39. [Crossref] [PubMed]
- Liu M, Ren Y, Zhou Z, et al. The crosstalk between macrophages and cancer cells potentiates pancreatic cancer cachexia. Cancer Cell 2024;42:885-903.e4. [Crossref] [PubMed]
- Martignoni ME, Dimitriu C, Bachmann J, et al. Liver macrophages contribute to pancreatic cancer-related cachexia. Oncol Rep 2009;21:363-9.
- Pizza FX, Peterson JM, Baas JH, et al. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J Physiol 2005;562:899-913. [Crossref] [PubMed]
- Olson B, Zhu X, Norgard MA, et al. Lipocalin 2 mediates appetite suppression during pancreatic cancer cachexia. Nat Commun 2021;12:2057. [Crossref] [PubMed]
- Deng M, Aberle MR, van Bijnen AAJHM, et al. Lipocalin-2 and neutrophil activation in pancreatic cancer cachexia. Front Immunol 2023;14:1159411. [Crossref] [PubMed]
- Deyhle MR, Callaway CS, Neyroud D, et al. Depleting Ly6G Positive Myeloid Cells Reduces Pancreatic Cancer-Induced Skeletal Muscle Atrophy. Cells 2022;11:1893. [Crossref] [PubMed]
- Deyhle MR, Hyldahl RD. The Role of T Lymphocytes in Skeletal Muscle Repair From Traumatic and Contraction-Induced Injury. Front Physiol 2018;9:768. [Crossref] [PubMed]
- D'Alessio FR, Kurzhagen JT, Rabb H. Reparative T lymphocytes in organ injury. J Clin Invest 2019;129:2608-18. [Crossref] [PubMed]
- Zhang J, Xiao Z, Qu C, et al. CD8 T cells are involved in skeletal muscle regeneration through facilitating MCP-1 secretion and Gr1(high) macrophage infiltration. J Immunol 2014;193:5149-60. [Crossref] [PubMed]
- Masuda S, Yamakawa K, Masuda A, et al. Association of Sarcopenia with a Poor Prognosis and Decreased Tumor-Infiltrating CD8-Positive T Cells in Pancreatic Ductal Adenocarcinoma: A Retrospective Analysis. Ann Surg Oncol 2023;30:5776-87. [Crossref] [PubMed]
- Zahm CD, Colluru VT, McIlwain SJ, et al. TLR Stimulation during T-cell Activation Lowers PD-1 Expression on CD8(+) T Cells. Cancer Immunol Res 2018;6:1364-74. [Crossref] [PubMed]
- Michaelis KA, Norgard MA, Zhu X, et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat Commun 2019;10:4682. [Crossref] [PubMed]
- Amitani M, Asakawa A, Amitani H, et al. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol 2013;45:2179-85. [Crossref] [PubMed]
- Hamauchi S, Furuse J, Takano T, et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 2019;125:4294-302. [Crossref] [PubMed]
- Takayama K, Takiguchi T, Komura N, et al. Efficacy and safety of anamorelin in patients with cancer cachexia: Post-hoc subgroup analyses of a placebo-controlled study. Cancer Med 2023;12:2918-28. [Crossref] [PubMed]
- Iwai N, Sakai H, Oka K, et al. Predictors of response to anamorelin in gastrointestinal cancer patients with cachexia: a retrospective study. Support Care Cancer 2023;31:115. [Crossref] [PubMed]
- Herodes M, Anderson LJ, Shober S, et al. Pilot clinical trial of macimorelin to assess safety and efficacy in patients with cancer cachexia. J Cachexia Sarcopenia Muscle 2023;14:835-46. [Crossref] [PubMed]
- Ahmed DS, Isnard S, Lin J, et al. GDF15/GFRAL Pathway as a Metabolic Signature for Cachexia in Patients with Cancer. J Cancer 2021;12:1125-32. [Crossref] [PubMed]
- Summerbell CD, Youle M, McDonald V, et al. Megestrol acetate vs cyproheptadine in the treatment of weight loss associated with HIV infection. Int J STD AIDS 1992;3:278-80. [Crossref] [PubMed]
- McMillan DC, O'Gorman P, Fearon KC, et al. A pilot study of megestrol acetate and ibuprofen in the treatment of cachexia in gastrointestinal cancer patients. Br J Cancer 1997;76:788-90. [Crossref] [PubMed]
- Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2013;2013:CD004310. [Crossref] [PubMed]
- Gold J, High HA, Li Y, et al. Safety and efficacy of nandrolone decanoate for treatment of wasting in patients with HIV infection. AIDS 1996;10:745-52. [Crossref] [PubMed]
- Storer TW, Woodhouse LJ, Sattler F, et al. A randomized, placebo-controlled trial of nandrolone decanoate in human immunodeficiency virus-infected men with mild to moderate weight loss with recombinant human growth hormone as active reference treatment. J Clin Endocrinol Metab 2005;90:4474-82. [Crossref] [PubMed]
- Wiedenmann B, Malfertheiner P, Friess H, et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J Support Oncol 2008;6:18-25.
- Gordon JN, Trebble TM, Ellis RD, et al. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut 2005;54:540-5. [Crossref] [PubMed]
- Reid J, Mills M, Cantwell M, et al. Thalidomide for managing cancer cachexia. Cochrane Database Syst Rev 2012;2012:CD008664. [Crossref] [PubMed]
- Mantovani G, Macciò A, Madeddu C, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200-11. [Crossref] [PubMed]
- Golan T, Geva R, Richards D, et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J Cachexia Sarcopenia Muscle 2018;9:871-9. [Crossref] [PubMed]
- Wigmore SJ, Ross JA, Falconer JS, et al. The effect of polyunsaturated fatty acids on the progress of cachexia in patients with pancreatic cancer. Nutrition 1996;12:S27-30. [Crossref] [PubMed]
- Riemersma RA. Essential Fatty Acids and Eicosanoids: Invited Papers from the Fourth International Congress. New York, NY, USA: The American Oil Chemists Society; 1998:456.
- Wigmore SJ, Barber MD, Ross JA, et al. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer 2000;36:177-84. [Crossref] [PubMed]
- Fearon KC, Barber MD, Moses AG, et al. Double-blind, placebo-controlled, randomized study of eicosapentaenoic acid diester in patients with cancer cachexia. J Clin Oncol 2006;24:3401-7. [Crossref] [PubMed]
- Fearon KC, Von Meyenfeldt MF, Moses AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 2003;52:1479-86. [Crossref] [PubMed]
- Murphy RA, Yeung E, Mazurak VC, et al. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br J Cancer 2011;105:1469-73. [Crossref] [PubMed]
- Abe K, Uwagawa T, Haruki K, et al. Effects of ω-3 Fatty Acid Supplementation in Patients with Bile Duct or Pancreatic Cancer Undergoing Chemotherapy. Anticancer Res 2018;38:2369-75. [Crossref] [PubMed]
- Wigmore SJ, Falconer JS, Plester CE, et al. Ibuprofen reduces energy expenditure and acute-phase protein production compared with placebo in pancreatic cancer patients. Br J Cancer 1995;72:185-8. [Crossref] [PubMed]
- Kouchaki B, Janbabai G, Alipour A, et al. Randomized double-blind clinical trial of combined treatment with megestrol acetate plus celecoxib versus megestrol acetate alone in cachexia-anorexia syndrome induced by GI cancers. Support Care Cancer 2018;26:2479-89. [Crossref] [PubMed]
- Bowers M, Cucchiaro B, Reid J, et al. Non-steroidal anti-inflammatory drugs for treatment of cancer cachexia: A systematic review. J Cachexia Sarcopenia Muscle 2023;14:2473-97. [Crossref] [PubMed]
- Chrysostomou SE, Eder S, Pototschnig I, et al. R-ketorolac ameliorates cancer-associated cachexia and prolongs survival of tumour-bearing mice. J Cachexia Sarcopenia Muscle 2024;15:562-74. [Crossref] [PubMed]
- Roeland EJ, Bohlke K, Baracos VE, et al. Management of Cancer Cachexia: ASCO Guideline. J Clin Oncol 2020;38:2438-53. [Crossref] [PubMed]
- Bach-Faig A, Berry EM, Lairon D, et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr 2011;14:2274-84. [Crossref] [PubMed]
- Bagheri A, Asoudeh F, Rezaei S, et al. The Effect of Mediterranean Diet on Body Composition, Inflammatory Factors, and Nutritional Status in Patients with Cachexia Induced by Colorectal Cancer: A Randomized Clinical Trial. Integr Cancer Ther 2023;22:15347354231195322. [Crossref] [PubMed]
- Gong J, Thomassian S, Kim S, et al. Phase I trial of Bermekimab with nanoliposomal irinotecan and 5-fluorouracil/folinic acid in advanced pancreatic ductal adenocarcinoma. Sci Rep 2022;12:15013. [Crossref] [PubMed]


