
Biliary complications after liver transplantation: current perspectives and future strategies
Introduction
Liver transplantation (LT) is a life-saving therapy for patients with end-stage liver disease or with acute liver failure, ensuring excellent outcomes and survival rates at 1 and 5 years (85–90% and 70%, respectively) (1). Biliary complications (BCs) are the most common complications after LT. Despite the improvements in surgical techniques, immunosuppressive regimens, and organ preservation, BCs remain an important source of mortality and morbidity, leading to long-term repeated therapies including endoscopic, percutaneous and surgical procedures. BCs incidence rate after LT is reported to range from 5% to 20%. Although most of them occur in the first three months, they may also appear several years after LT (2).
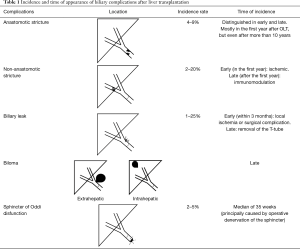
Between BCs, the most frequent are anastomotic strictures (ASs), non-anastomotic strictures (NAS), and biliary leakage (Figure 1 and Table 1). Ischemic-type biliary lesions, sphincter of Oddi dysfunction (SOD), haemobilia and biliary obstruction by cystic duct mucoceles, stones, sludge, or casts are observed less frequently (3).
Full table
Therapeutic options include endoscopic techniques [i.e., endoscopic retrograde cholangiopancreatography (ERCP)], percutaneous trans-hepatic biliary drainage (PTBD) and surgery techniques. While ERCP represents the first-line treatment in most of cases, PTBD is usually performed in patients with a Roux-en Y hepaticojejunostomy, a kind of biliodigestive anastomosis that makes ERCP technically difficult. Surgery is reserved to patients who failed endoscopic or percutaneous approaches.
The aim of this review is to summarize the available evidences on pathophysiology, risk factors, diagnosis and therapeutic management of BCs after LT. We present the following article in accordance with the Narrative Review reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.09.01/rc).
Risk factors
BCs could be related to several risk factors (see Figure 2), such as the reconstruction technique, the use of biliary splintage, the type of LT procedure, the organ preservation, the chronic rejection, the hepatic artery (HA) thrombosis and other recipient and donor characteristics.
Prevention of BCs is not simple and it is mainly related on the perioperative management of patients. Many preventive strategies are still debated, needing further studies. It is important to ensure a valid blood supply to the bile duct during all the phases of transplantation, of both donor and recipient. Any damage to the graft bile ducts should be strictly avoided and any tiny bleeding requires suture ligation with fine needles. Moreover, in a duct-duct reconstruction, it is important to leave enough length of the bile ducts for a tension-free anastomosis. There is still also a great debate on which type of anastomosis could be better and no prospective trials are available.
Moreover, a recent systematic overview of 45 papers (14,411 subjects) found that the patients more susceptible of BCs are those with MELD >25, primary sclerosing cholangitis (PSC) or malignancies (4).
Immunological risk factors
Cytomegalovirus (CMV) infection may increase the risk to develop BCs after LT. The interrelation of CMV with human leukocyte antigens (HLA) has been studied as possible risk factor for the vanishing bile duct syndrome and these findings would be consistent with precipitation of chronic rejection by CMV-induced HLA expression in patients rendered susceptible by the donor/recipient HLA antigens match (5). Halme et al. (6) showed that BCs after LT occur more often in patients with preceding or concomitant CMV viremia, and are common especially in association with primary CMV infection. For these reasons, CMV prophylaxis is recommended for liver recipients who are seronegative for CMV, to prevent the development of BCs.
Donor and recipient characteristics
The donor risk index is a score index that includes seven donor’s variables that could independently predict an increased risk of graft failure: donor age over 40 years (particularly over 60 years), donation after cardiac death (DCD), and split/partial grafts were strongly associated with graft failure, while African-American race, low body weight, cerebrovascular accident and other causes of brain death were more modestly, but still significantly, associated with graft failure (7).
A case-control study evaluated transplanted patients with grafts from donors who were 75 years or older in age, showing that the donor’s age (>75 years) was not associated with the occurrence of BCs after LT, although these results were observed in a small cohort of patients (8).
Graft macrovascular steatosis >25% is an independent risk factor predicting the occurrence of BCs. It has been histologically demonstrated that fat infiltration of the liver is associated with a decrease of hepatic sinusoid space by 50% compared with a normal liver, and it may reduce the total hepatic flow leading to ischemic biliary lesions (9).
Regarding recipient characteristics, it was observed that in the post-MELD era, after 2002, biliary strictures were more common compared to the past (15.4% versus 6.4%, P<0.001), independently of surgical techniques, suggesting that the patients that underwent LT began to be sicker in the last years (1).
Surgical techniques risk factors
Biliary reconstruction over a T-tube used to be the gold standard technique in most transplant centers, but it is also a source of controversy, as only few randomized trials exist on this topic. Weiss et al. evaluated the benefit and the risks of the usage of T-tubes, demonstrating a decreased rate of BCs in patients treated with T-tube insertion in comparison with those where T-tube has not been used (27% vs. 50%) (10). Two systematic reviews and meta-analyses (11,12). showed an increased occurrence of biliary strictures with the use of T-tube, but no differences in terms of biliary leaks. The authors concluded that there was no evidence in favor of the use of a T-tube. Finally, in a prospective randomized trial by López-Andújar et al. (13), 187 liver recipients were assigned to choledochostomy with or without T-tube, reporting a similar overall BCs rate between the two groups with a reduced incidence of AS and severe BCs in the T-tube group.
Regarding the type of anastomosis, some retrospective studies (14-17) showed that in deceased donor LT (DDLT) the success rate is the same between duct-to-duct and hepaticojejunostomy anastomosis, even if there is a higher incidence of bacterial colonization, bleeding and leaks in the latter (18). Anyway surgeons should follow the principle of tension-free and viable anastomosis to choose the best type of anastomosis.
Suture technique and materials have also a potential impact on the risk of BCs after LT, although the optimal technique of reconstruction remains unclear. A consensus on type of suture is also still lacking: some groups prefer biliary anastomosis with continuous absorbable sutures (19), some others instead interrupted sutures with non-absorbable monofilaments (20). A retrospective cohort study compared the rate of BCs between interrupted and continuous suture techniques for end-to-end biliary anastomosis, showing no significant differences in BCs and graft or patients survival between the two techniques (21). Similarly, no differences in BCs were observed comparing a biliary reconstruction performed completely by continuous suture technique versus a mixed technique in which the posterior wall of the bile duct was closed by continuous technique and the anterior wall was closed by interrupted suture technique (22). However, the retrospective nature of these data do not allow definitive conclusions to be reached. Finally, more evidences are needed to assess the use of the T-tube or the new strategies in terms of surgical techniques that may reduce the incidence of BCs.
Etiology of BCs
ASs
By definition, ASs are isolated strictures localized within one centimeter of the surgical anastomosis and their incidence is about 4–9% after LT (23). Depending on the time of the occurrence, they can be distinguished in early and late. Early ASs occur in the first three months after LT and they are mostly related to the size mismatch between donor’s and recipient’s bile duct and to the presence of edema; late ASs occur three months after LT and they can be due to local ischemia and fibrosis (24). A systematic review showed that ASs can occur very early after LT (even after only 7 days), but also 11 years later (3). Generally the most common time of appearance is within one year. Technical issues are the most important risk factors for ASs, like improper surgical technique, small caliber of the bile ducts, inappropriate suture material, tension at the anastomosis, and infection (25). Verdonk et al. (23) assessed the impact of the occurrence of ASs on patient and graft survival, showing no significant differences between patients with or without ASs; however, median time of follow up was 3.6 years and probably it was not long enough if we consider that LT should give a benefit in term of survival of 5 years.
Biliary leakages (BLs)
BLs consist in a leakage originating from the bile duct or sometimes from cystic duct with or without the formation of a biloma. They could complicate from 1% to 25% of performed LT (26). A meta-analysis (3) documented postoperative biliary leakage in 936 on 11,397 patients (8.2%), 7.8% (668/8,585) among DDLT patients and 9.5% (268/2,812) among living donor LT (LDLT) recipients; the onset of biliary leakage ranged from 1 day to 6 months after transplantation. Early BLs occur within 3 months and are often caused by local ischemia due to the necrosis at end of the bile duct (donor duct) or related to surgical procedures. An important risk factor is receiving a graft from split liver or from a LDLT. In this case, they usually occur from the cut surface of the partial liver or from disruption of the surgical biliary anastomosis. A rare but serious acute event in the early post operatory timing is diffuse biliary necrosis secondary to acute arterial thrombosis, and the clinical presentation is usually massive bile leakage, sepsis, cholestasis (27,28). Late BLs are often related to the removal of the T-tube and occur at the T-tube insert site; the incidence of late BLs was reported to be 7% with a mean time of presentation of 118 days after LT. Clinically, BLs could be totally asymptomatic and sometimes they are only detected during routine abdominal ultrasound or cross sectional imaging (29).
NASs
NASs are defined as strictures or irregularities in the biliary tree beyond one centimeter from the surgical anastomosis. They are frequently intrahepatic and they may be associated with the formation of biliary casts or stones. One of the most important risk factors is HA thrombosis, that can be observed in about 50% of patients affected by NAS (29). Early studies described prolonged cold ischemia times above 10 hours, bile-salt toxicity, immune-mediate injury (i.e., AB0 incompatibility), and ischemic reperfusion injury as other important risk factors for development of NASs (30). Akamatsu et al. showed that NASs develop in 2–20% of patients and that they are localized in proximity of the anastomosis and occur in the presence of a normal vascular situation. These biliary alterations resemble to the ischemic lesions of the biliary tree, and they have been referred to ischemic type biliary complications (ITLBs) (3,31).
The bile ducts epithelium consisted of cholangiocytes, and its vascularization depends on blood flow from the HA (32). For this reason, every damage on HA during procurement or reconstruction can be related to ITLBs. Furthermore, among the hepatic cells, cholangiocytes seem to be the more sensitive to ischaemia compared to hepatocytes or Kuppfer cells, as observed in vitro studies (33). During liver recruitment the whole blood flow is interrupted for several hours and it could cause ischemic lesions, evidencing how the prolonged cold ischaemic time (CIT) and ischaemia reperfusion (IR) play a key role on the occurrence of Ischaemic cholangiopathy (IC) (34). Chan et al. showed that a CIT longer than 9 hours increases incidence of IC (RR: 2.7; P=0.013) (35). Finally, during the re-vascularization, the oxidative stress produced might activate Kuppfer cells with mild initial injury and it could progress in a more serious injury with immune activation (36).
The occurrence of NASs depends also on which type of graft is transplanted. The incidence is major in DCD (30–50%) in comparison with donation after brain death (DBD) (4–15%) (37).
Outcomes of DCD recipients after LT are worse than DBD recipients, especially in terms of rate of overall BCs (29% vs. 17%) (38). The warm ischemia time represents a crucial issue: for DCD livers it exists a second warm ischemia time corresponding to time from circulatory arrest until the preservation that may contribute to development of IC. Jay et al. described also a worst patients survival compared to DBD (P<0.001): 1- and 3-year survival was 82% and 71% for DCD vs. 86% and 77% for DBD recipients. Moreover, combination of DCD with cold ischemia time >12 hours (HR =1.81), shared organs (HR =1.69), recipient hepatocellular carcinoma (HR =1.80), recipient age >60 years (HR =1.92), and recipient renal failure (HR =1.82) were associated with an increased risk of mortality (39).
Regarding the surgical techniques, the American Society of Transplant Surgeon recommends a limit of WIT between 20 to 30 min (40). Taner et al. showed that the asystole-to-cross clamp duration [odds ratio (OR):161, 95% confidence interval (CI): 1.021–1.321] and African American recipient race (OR: 5.374, 95% CI: 1.368–21.103) were significant predictors for the development of IC (P<0.05) (41). Finally, in an era where scarcity of liver grafts is a consistent issue, it would be necessary to select and to match best donor grafts in the case of DCD liver transplantation to avoid the occurrence of BCs and graft loss.
Buis et al. (42) showed that NASs in the first year is related to ischemic events, while NAS after the first year is associated with immunomodulation. Regarding the immuno-mediate process, an increased risk for NASs is present in patients with ductopenic rejection, concomitant CMV infection or in those who receive an AB0 blood type-mismatched graft.
Patients with PSC are at higher risk of developing post-transplant strictures. Over the classic risk factors for NAS, these patients have a slightly risk of recurrence of PSC with a variable prevalence depending on different studies from 6% to 37% (43,44). This makes more challenging the diagnosis of late strictures in the PSC subgroup of liver-transplanted patients.
SOD
After LT, donor’s and recipient’s bile duct dilatation is common, also without any clinical or biochemical abnormality. In 2–5% of liver transplant patients, this event could be accompanied by a biochemical alteration in the absence of cholangiography evidence of obstructions, and is due to SOD. It is principally caused by operative denervation of Sphincter of Oddi resulting in abnormal ampullary relaxation. The onset time is reported to be 35 weeks and the principal risk factor is the insertion of a T-tube (45).
Biloma
Biloma formation is secondary to an extravasation of bile into the intrahepatic parenchyma of free abdominal cavity. A severe complication is bile duct necrosis with subsequent bile duct rupture. Bilomas may be also infected leading to abscesses and sepsis, but the most serious complication is the erosion of the hepatic artery (46).
Filling defects
Biliary stones can appear at any time after LT but they are more common as late complication occurring later than 3 months after LT (47). Principal risk factors are the presence of biliary strictures, mucosal damage, ischemia, infection foreign bodies (T tube or stents). Sludge can also occur without any of these risk factors. Finally, treatment with calcineurin inhibitors may contribute to biliary stone formation (48).
Clinical and diagnosis
Clinical presentation of BCs after LT is heterogeneous, consisting in a wide spectrum of physical, biochemistry or radiological abnormalities. In some cases, only asymptomatic cholestasis [increasing gamma-glutamyltransferase (GGT) and/or alkaline phosphatase (ALP) levels] can be present, while other patients can show a more challenging clinical presentation with severe cholangitis and biliary peritonitis. More frequently, symptoms of BCs are unspecific and can be associated with jaundice or with other signs of cholestasis. The main differential diagnoses are hepatic artery occlusion, rejection, sepsis and post-transplant hepatitis. A prospective study conducted on 56 consecutive liver transplant recipients developing fever or infections in the Intensive Care Unit showed that the biliary tree was the third source of the infections associated with fever in 9% of cases, preceded by pneumonia and catheter-related bacteremia (49).
Patients with a suspicion of BCs should underwent to abdominal ultrasound (US) as first step, to assess the presence of dilated bile ducts, to show the presence of abscesses and to exclude hepatic artery occlusion by Doppler (Figure 3). In a retrospective single-centre study of 79 liver transplanted patients with choledococholedocostomy, the predictive value of US was assessed in comparison with ERCP, the gold standard for the diagnosis of biliary leaks or strictures, showing a sensitivity of 77%, and specificity of 67%, with positive and negative predictive values of 26% and 95% respectively (50) Considering that the prevalence of BCs after liver transplant was relatively low (13%), these results suggest that a negative US makes the presence of BCs unlikely. If US does not allow the exclusion of hepatic artery occlusion, a computed tomography (CT)-angiography should be performed to assess more accurately the patency of hepatic artery and to exclude the presence of collections. If CT is negative in this sense, the morphology of biliary tree has to be assessed with more sensitive diagnostic modalities, such as magnetic retrograde cholangiopancreatography (MRCP) or direct cholangiography [with ERCP or percutaneous trans-hepatic cholangiography (PTC)].
Direct cholangiography techniques represent the gold standard for evaluating the biliary tract, but they are invasive and the risk of serious complications such as bleeding, perforation, sepsis and death ranges from 1% to 10% (51,52). For these reasons, today MRCP, allowing the same detailed visualization of the biliary ducts to be generated non-invasively, is considered an accurate and safe alternative to invasive cholangiography and it does not need the administration of intravenous contrast agent. Several studies (53-59) assessed the diagnostic value of MRCP suggesting that MRCP is a reliable technique for the diagnosis of intra- and extrahepatic BCs, with similar detection rates in comparison with ERCP and PTC (see Table 2). On the basis of these data, nowadays MRCP is recommended to plan and guide therapeutic interventions (PTC, ERCP or surgery) while invasive cholangiography should be restricted for therapeutic uses or in the cases in which MRCP is equivocal. MRCP is effective in the identification of ischemic-type biliary lesions, of anastomotic and non-anastomotic strictures and of stones, leading to a significant reduction of the number of diagnostic ERCP (57). MRCP is conventionally performed without intravenous contrast agent, using T2-weighted sequences, although more recently an Italian study assessed the effectiveness of contrast-enhanced T1-weighted sequences, showing an improvement of diagnostic confidence, compared to conventional MRCP (60). Furthermore, MRCP is the preferred diagnostic technique in patients with Roux-en-Y hepaticojejunostomy, in which ERCP could be technically difficult. However, a retrospective study of 50 patients with Roux-en-Y anastomosis evaluated both diagnostic and therapeutic effectiveness of ERCP using a single-balloon enteroscope, showing promising results (61).

Full table
Treatment
BCs after LT, from both living or deceased donor, can be managed in a conservative, radiological, endoscopic or surgical way, or with a combination of these. In the last two decades, endoscopy became the technique of choice of BCs treatment and it represents the first line treatment with a success rate of 70–100%. The ERCP procedure is much more challenging in post-LDLT compared to DDLT recipient for size discrepancy between donor and recipient ducts and because the anastomosis is higher and peripheral, making the access more difficult. ERCP is generally considered a safe procedure, even if some complication can occur, with a range varying between 5% (62) and 10% (63) of cases, in relationship with the complexity of the intervention, patient’s characteristics and endoscopist’s expertise. It is difficult to estimate the risk of complications after an ERCP procedure in patients underwent LT, mostly because the studies available are few and retrospective (64-68). The most frequent post-ERCP complications, with a wide range of incidence, were bleeding [1.65% (64)–8.5% (66)], acute pancreatitis [2.7% (67)–6.4% (66)] and cholangitis [0.7% (65)–5.1% (68)]. Perforation was rare, with an estimated incidence of 0.06% (68).
When endoscopy is not able to treat the BCs, mainly because of impassable stenosis, intrahepatic/hilar stenosis or anatomical variations (both natural or post-surgical), the radiological or the surgical approaches can be adopted. Radiological approach consists in a percutaneous transhepatic biliary drainage, which can be performed alone or combined with endoscopy (rendezvous procedure). The combined success rate of ERCP and PTBD overcome 90% of cases. In the small number of patients in which both ERCP and PTBD have failed, surgical intervention can be performed (69). When no interventional strategy can be performed because of the presence of anatomical difficulties (hepatic artery thrombosis), ischemic cholangitis, excessive fragility of biliary system, or recurrent cholangitis despite rotating antibiotic therapy in patients with NAS, the only rescue therapy is the re-transplantation.
Biliary strictures
For years, the most frequently performed method to treat biliary strictures has been balloon dilatation. This technique consists in the insertion, after sphincterotomy (or alternatively a precut), of an inflatable balloon. Balloon dilatation alone has been progressively abandoned because of the high percentage of restenosis (up to 47%) (70,71) and also because of the high risk of rupture of the biliary tract. For this reason, pneumatic balloon dilatation should be reserved only for the first endoscopic procedure. In 2006, Zoepf et al. (72) showed that the association of balloon dilatation with the insertion of a plastic protheses was more effective than balloon dilatation alone, both in terms of success rate and stenosis recurrences.
With the increased development and improvement of endoscopic instruments, it has been possible to treat more aggressively the benign biliary strictures (BBS). In 2001 Costamagna et al. (73) assessed the technique of balloon dilatation in association with a multiple plastic stent (MPS) insertion in a single center experience. This technique consists in the placement, after a standard balloon dilatation, of an increasing number of plastic biliary stents, until the complete disappearance of the BBS. On an intention-to-treat analysis, the success rate for this endoscopic treatment had been 89%. After that, many articles were published regarding this technique (65,72,74-84) and in 2017 Koksal et al. (85) summarized these results in a review: a higher resolution rate and a lower recurrence rate were showed in patients with more than 12 months of stenting, with higher total number of stents and number of stents inserted per session.
An alternative treatment for biliary strictures is the insertion of metal stents, in particular covered metal stents. Uncovered metal stents can lead to reactive tissue hyperplasia, especially around the anastomosis, complicating the subsequent removal, and for this reason, they should not be used (86). Fully covered self-expandable metal stents (FC-SEMS) have the advantage, respect to plastic stents, of not repeating ERCP to place multiple stents (72,73,87-101). The aforementioned review (75) reported a stricture resolution rate between 53% and 100% and a recurrence rate up to 50%. The response rate and the recurrence rate of patients in relationship to the duration of stenting (less or more than three months) were respectively 70.4% (93/132) vs. 78.7% (189/240) and 32.6% (31/95) vs. 30% (30/100). In 2017, a meta-analysis of 22 studies (18 observational studies and 4 randomized trials) including 1,298 patients assessed the overall stricture resolution rate, recurrence rate, and the safety of FC-SEMSs in BBS treatment (102). Weighted pooled BBS resolution rate and stricture recurrence rate with FC-SEMS were 83% and 16%, respectively; overall rate of adverse events requiring intervention and/or hospitalization was 15%. However, some biases affected this meta-analysis (103), as the poor representativeness of the patients in comparison to real epidemiology of BBS. In 2018 a new meta-analysis has been published to compare MPS and SEMS exclusively in the treatment of biliary strictures after LT (104), including three randomized controlled trials and one retrospective cohort study (179 patients treated with MPS and 119 patients treated with SEMS). This meta-analysis, based on low-quality evidence, showed an advantage of SEMS in terms of the number of ERCP procedures (mean difference: 1.69) and treatment days (mean difference: 40.2 days), without differences in terms of biliary stricture resolution or recurrence rate.
Magnetic compression anastomosis (MCA) is an alternative technique, in which a magnet is placed to the proximal site of the stricture percutaneously and a second magnet is placed to the distal site of the stricture endoscopically. The approximation of the magnets causes necrosis of the tissue between them and creates a fistula which enables to traverse a guidewire (105). Data on efficacy are still lacking, but a recent case series in which MCA was realized on benign biliary strictures (nine patients were included and six of these had undergone LT) showed a successful recanalization. However, it is needed to underline that after recanalization multiple plastic stents, fully covered SEMS or PTBD were used. Therefore, further studies are needed to validate this approach for biliary strictures (106).
Treatment of NAS
The management of NAS is more complex in comparison with AS and a high number of patients undergo re-transplantation because of this complication (107). Endoscopic treatments have a success rate in NAS variable from 50% to 75% (108). This is due to the fact that NAS are more often multiple, intrahepatic of the small ducts or quite extended, making difficult to be reached endoscopically. So, patients more susceptible of endoscopic therapies like stenting or balloon dilatation, are those with NAS of the common bile duct or of the left/right hepatic duct. Moreover, if endoscopic therapy is feasible, this often requires a higher number of interventions (7 vs. 3) and longer treatments (185 vs. 67 days) (18). In LDLT the rate of endoscopic success is even lower. The development of NAS strictures reduces graft survival, although it does not decrease patient survival (109). In patients with narrow stenosis or intrahepatic small ducts NAS, PTC has more possibilities of success. NAS secondary to early hepatic artery thrombosis require urgent revascularization or re-transplantation. Diffuse disease in the smaller intrahepatic ducts is a reason for considering early repeat transplantation.
Biliary leaks
Small collections resolve spontaneously whereas larger collections can cause mass effect or become secondarily infected (105). Non-ischemic leaks usually respond to non-operative diversion of biliary flow, such as unclamping of the T tube, endoscopic sphincterotomy or PTC. Frequently, a simple drainage through the tube opening could be therapeutic, preventing any invasive treatment. In other cases, endoscopic treatment should be the first line of care. A sphincterotomy alone could be therapeutic in case of small bile leak (110) while the positioning of a stent could be necessary for major bile leaks (111). In these patients, the stent is left in place for 2–3 months and the success rate is about 95%. When endoscopic cannulation of the duct is not possible (e.g., in Roux-en-Y patients), an internal-external biliary drainage catheter can be placed with a percutaneous approach. An alternative is represented by the rendezvous technique, in which the interventional radiologist places a catheter via a percutaneous approach, and subsequently, the endoscopist proceed to the stenting (105). Re-operation and re-transplantation for bile leak can be an alternative (3) but, with the improvements in radiologic and endoscopic techniques, this option is rarely performed.
Biloma
The majority of these fluid collections are small and asymptomatic and usually resolve without intervention (95%) (112), especially if they communicate with the biliary tree. The standard treatment in more complex cases is the radiological percutaneous drainage and antibiotics (46). Some cases can require placement of a biliary stent in the extrahepatic bile duct (113). If bile leaks cannot be effectively controlled with the abovementioned treatments, surgery is indicated.
SOD
To date, no clinical trials assessing the treatment for SOD are available in patients with BCs after LT. Patients with a suspected SOD often undergo ERCP with manometry and sphincterotomy with variable success. In the EPISOD trial (114), 214 patients with post-cholecystectomy pain and suspected SOD were randomized to sphincterotomy or sham therapy, and no differences were found between the two groups. In 2018, the EPISOD II trial (115) reporting EPISOD patients outcomes up to 5 years, confirmed the results previously showed.
Filling defects
Also in this case the treatment of choice is endoscopy, with various combination of sphincterotomy, balloon and basket extraction, stent placement or lithotripsy. In many cases, especially for biliary casts, endoscopy is associated with treatment failure and PTC can be used (116).
Future perspectives
The various scenarios of BCs after LT and their important clinical issues have led scientific research to explore possible other etiologies and treatments to reduce their prevalence end their impact on the overall and organ survival.
Immunological features: the role of anti-DSA
Regarding the immunological features, the role of donor specific alloantibodies (DSA) is unclear. These antibodies play a main role in kidney transplantation, in acute and chronic antibody mediated rejection (AMR) induction with early and late graft loss (117).
Regarding BCs after LT, a recent study (118) examined the role of DSA in NASs. Overall, 68 patients with NASs and 83 controls were included in this study. The development of DSA post-transplantation was not related to NASs development, as 26.5% of NASs patients and 16.9% of the controls had de novo DSA 1 year after LT (P=0.15). In conclusion, time-dependent regression analysis identified both NASs (adjusted HR 8.05, CI: 3.28–19.77, P<0.01) and de novo class II DSA (adjusted HR 2.84, CI: 1.38–5.82, P<0.01) as independent risk factors for graft loss. Preformed or de novo DSA were not associated with the development of NASs. However, NASs as well as de novo class II DSA were independent risk factors for graft loss after LT.
A multicenter cohort study in the Scandiatransplant organ sharing organization region is recruiting patients to analyze the impact of DSA on all-cause mortality and re-transplantation, early allograft dysfunction, acute and chronic rejection, fibrosis, vascular, and BCs. The primary aims of this prospective study are to investigate if DSA both pre-formed, persistent, and de novo affect survival and allograft loss including BCs and to evaluate the role of alloimmunisation. A prospective study is now already recruiting (Immunisation Anti HLA in the Liver Transplant Recipients: DSATH, clinical trials.gov) and it will analyze immunisation markers at the time of LT and systematically during follow-up. This could allow to characterize the histological and biliary lesions due to humoral immunisation.
The role of anti DSA remains substantially controversial in LT and its complications and more studies are needed to assess their impact on early and late outcomes.
Donor’s features: machine perfusion (MP) and the new era of defatting
Donor’s features represent important open issues considering the increased demand of liver donors and the lack of organs. For these reasons, many LT centers decided to turn to marginal allografts from extended criteria donors and donation after cardiac death. Their use increases the risk of primary non function, early graft dysfunction, BCs [particularly NAS (119,120)], decreased long-term graft survival (121). The primary objective of preservation is to attenuate ischemia/reperfusion injury, but marginal livers tolerate ischemia poorly (122). The biliary system is richly vascularized, and severe hypotension in organ donors causes microcirculatory dysfunctions that may led to an ischemic cholangiopathy with biliary necrosis and multifocal stenosis. For this reason, optimal organ preservation is essential for a low biliary morbidity (123).
Static cold storage (SCS) remains the standard of care preservation method in LT. Normothermic machine perfusion (NMP) maintains the liver ex vivo in a fully functioning state: its potential has been assessed showing a good safety end feasibility, although an improvement in patient or graft survival, or a reduction in BCs rate were not observed (124,125). Hypothermic machine perfusion (HMP) is an emerging technology that limits the ischemia/perfusion injury sustained during allograft preservation (126). Regarding the incidence of BCs, the first prospective trial on this issue compared 20 liver allografts underwent HMP with a matched group of SCS liver allografts, showing a reduction in BCs in the first group (127). Dutkowski et al. conducted a multicenter trial evaluating hypothermic oxygenated perfusion techniques in DCD liver allografts: a significant reduction in the rate of BCs and an improved 1-year graft survival were observed in the HMP-CD group compared with the SCS-DCD group (128).
Guarrera et al. showed in the context of “orphan livers” (that are organs discarded), that the use of HMP improves the rate of BCs compared to the SCS liver allografts (P=0.001). The incidence of biliary strictures was 10% in HPM allografts vs. 33% in SCS cases (P=0.031) (129). Moreover Schlegel et al. evaluated the efficacy of the hypothermic oxygenated perfusion (HOPE) in patients receiving DCD liver transplant: 5-year graft survival, censored for tumour death, was 94% for HOPE-treated versus 78% in untreated patients (P=0.024) (130), suggesting that a simple end-ischemic reperfusion is effective and safety end it may be used in the field of extended DCD liver grafts.
Currently, clinical trials assessing a combination of NMP and HMP are lacking, but the continued development of ex vivo liver machine is essential to increase the use of liver allografts. Furthermore, a recent systematic review on the impact of MP on BCs after LT showed that MP was associated with better post-operative results in the incidence of ITLB, with a similar incidence in terms of biliary strictures and biliary leakages. In the future, randomized clinical trials assessing BCs occurrence as primary outcome are needed to clarify the impact of machine perfusion on outcomes after LT (131).
Another important issue regarding liver donors is the increased lipid content that is associated with worst graft outcomes post-transplant. The wide spreading of obesity has led to a very high prevalence of steatosis in donor organs that ranges from 13% to 28% (132), and moreover it was one of the most common reason for declining the liver in 39% of cases (133).
To expand the organ pool, many strategies were investigated, such as reducing steatosis with MP or with the defatting process during normothermic perfusion (134,135).
Up to date, there are many studies upon animal models, and the extrapolation of these to clinical human setting is needed to validate these future strategies.
Surgical techniques: use of biodegradable stent
The use of a biodegradable bile duct stent may solve the problem of its removal and the occurrence of biliary leaks. In different animal models, especially in pigs, the feasibility of the placement of a polylactide-barium sulfate bio-degradable biliary stent was tested. Laukkarinen et al. reported that the biodegradable stents disappeared in all transplanted pigs after 6 months and it dissolved safely (136,137). In the clinical human setting, the first randomized study was performed in 2016 by Janousek et al. in ten patients. The role of a stent made of machine-knitted polydioxanone monofilaments was assessed. The results showed that duct-to-duct biliary reconstruction using an absorbable internal stent had good patency in all 5 patients, without BCs (138). However, it was only a first experience with a very small sample size and further larger prospective randomized studies are needed to estimate the validity of this kind of stent.
Conclusions
BCs remain the most common complications after LT, both in the early post-operative period and in the long-term period, representing nowadays, an important source of mortality and morbidity. Principal risk factors include surgical techniques and donor’s characteristics for biliary leakage and anastomotic biliary strictures and vascular alterations for non-anastomotic biliary strictures. For NASs, the role of immune-mediate mechanisms involved in their development, especially the apparition of anti-DSA, is not completely known. This review focused on the heterogeneity of etiology, risk factors and clinical presentation of BCs after LT. Regarding the diagnosis, MRCP is the gold standard both for intra- and extrahepatic BCs, while invasive cholangiography should be restricted for therapeutic uses or when MRCP is equivocal. About treatment, endoscopic techniques are the first line of treatment with success rates of 70–100%. The combined success rate of ERCP and PTBD overcome 90% of cases. For biliary strictures, the endoscopic treatment concerns the placement of multi plastic stent or metallic stent, but there are no available evidences on which is the best technique. Biliary leaks often resolve spontaneously, or with the positioning of a stent in ERCP for major bile leaks. Finally, more evidence is needed to identify novel risk factors, to understand if there is an immunological status associated with the development of BCs and to prove the efficacy of the emerging surgical and machine perfusion techniques.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.09.01/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2019.09.01/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sundaram V, Jones DT, Shah NH, et al. Posttransplant biliary complications in the pre- and post-model for end-stage liver disease era. Liver Transpl 2011;17:428-35. [Crossref] [PubMed]
- Gastaca M. Biliary Complications after Orthotopic Liver Transplantation: A Review of Incidence and Risk Factors. Transplant Proc 2012;44:1545-9. [Crossref] [PubMed]
- Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int 2011;24:379-92. [Crossref] [PubMed]
- Nemes B, Gámán G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol 2015;9:447-66. [Crossref] [PubMed]
- O’Grady JG, Alexander GJ, Sutherland S, et al. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet 1988;2:302-5. [Crossref] [PubMed]
- Halme L, Hockerstedt K, Lautenschlager I. Cytomegalovirus infection and development of biliary complications after liver transplantation1. Transplantation 2003;75:1853-8. [Crossref] [PubMed]
- Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics Associated with Liver Graft Failure: The Concept of a Donor Risk Index. Am J Transplant 2006;6:783-90. [Crossref] [PubMed]
- González-Sánchez MR, Cascales-Campos PA, López-Espín JJ, et al. Donors Older Than 75 Years Do Not Influence the Appearance of Biliary Complications After Liver Transplantation. Transplant Proc 2018;50:640-3. [Crossref] [PubMed]
- Baccarani U, Isola M, Adani GL, et al. Steatosis of the hepatic graft as a risk factor for post-transplant biliary complications. Clin Transplant 2010;24:631-5. [Crossref] [PubMed]
- Weiss S, Schmidt SC, Ulrich F, et al. Biliary Reconstruction Using a Side-to-Side Choledochocholedochostomy With or Without T-Tube in Deceased Donor Liver Transplantation. Ann Surg 2009;250:766-71. [Crossref] [PubMed]
- Sotiropoulos GC, Sgourakis G, Radtke A, et al. Orthotopic Liver Transplantation: T-Tube or Not T-Tube? Systematic Review and Meta-Analysis of Results. Transplantation 2009;87:1672-80. [Crossref] [PubMed]
- Riediger C, Müller MW, Michalski CW, et al. T-tube or no T-tube in reconstruction of the biliary tract during orthotopic liver transplantation - systematic review and meta-analysis. Liver Transpl 2010;16:705-17. [Crossref] [PubMed]
- López-Andújar R, Orón EM, Carregnato AF, et al. T-tube or No T-tube in Cadaveric Orthotopic Liver Transplantation. Ann Surg 2013;258:21-9. [Crossref] [PubMed]
- Qian YB, Liu CL, Lo CM, et al. Risk factors for biliary complications after liver transplantation. Arch Surg 2004;139:1101-5. [Crossref] [PubMed]
- Thethy S, Thomson BNJ, Pleass H, et al. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant 2004;18:647-53. [Crossref] [PubMed]
- Sawyer RG, Punch JD. Incidence and management of biliary complications after 291 liver transplants following the introduction of transcystic stenting. Transplantation 1998;66:1201-7. [Crossref] [PubMed]
- Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg 1994;219:40-5. [Crossref] [PubMed]
- Graziadei IW, Schwaighofer H, Koch R, et al. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl 2006;12:718-25. [Crossref] [PubMed]
- Ishiko T, Egawa H, Kasahara M, et al. Duct-to-duct biliary reconstruction in living donor liver transplantation utilizing right lobe graft. Ann Surg 2002;236:235-40. [Crossref] [PubMed]
- Liu CL, Lo CM, Chan SC, et al. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation 2004;77:726-32. [Crossref] [PubMed]
- Castaldo ET, Pinson CW, Feurer ID, et al. Continuous versus interrupted suture for end-to-end biliary anastomosis during liver transplantation gives equal results. Liver Transpl 2007;13:234-8. [Crossref] [PubMed]
- Jafari A, Stoffels B, Kalff JC, et al. An Improved Suture Technique for Perform Biliary Reconstruction in Orthotopic Liver Transplantation. Ann Transplant 2016;21:25-9. [Crossref] [PubMed]
- Verdonk RC, Buis CI, Porte RJ, et al. Anastomotic Biliary Strictures After Liver Transplantation: Causes and Consequences. Liver Transpl 2006;12:726-35. [Crossref] [PubMed]
- Jagannath S, Kalloo AN. Biliary Complications After Liver Transplantation. Curr Treat Options Gastroenterol 2002;5:101-12. [Crossref] [PubMed]
- Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: A changing landscape of the Achilles’ heel. Liver Transpl 2006;12:702-4. [Crossref] [PubMed]
- Wojcicki M, Milkiewicz P, Silva M. Biliary Tract Complications after Liver Transplantation: A Review. Dig Surg 2008;25:245-57. [Crossref] [PubMed]
- Moser MA, Wall WJ. Management of biliary problems after liver transplantation. Liver Transpl 2001;7:S46-52. [Crossref] [PubMed]
- Verdonk RC, Buis CI, Porte RJ, et al. Biliary complications after liver transplantation: A review. Scand J Gastroenterol Suppl 2006;41:89-101. [Crossref] [PubMed]
- Egawa H, Inomata Y, Uemoto S, et al. Biliary anastomotic complications in 400 living related liver transplantations. World J Surg 2001;25:1300-7. [Crossref] [PubMed]
- Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl 2008;14:759-69. [Crossref] [PubMed]
- Fisher A, Miller CM. Ischemic-type biliary strictures in liver allografts: The achilles heel revisited? Hepatology 1995;21:589-91. [Crossref] [PubMed]
- Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol 2006;44:806-17. [Crossref] [PubMed]
- Cutrin JC, Cantino D, Biasi F, et al. Reperfusion damage to the bile canaliculi in trans- planted human liver. Hepatology 1996;24:1053-7. [Crossref] [PubMed]
- Sankary HN, McChesney L, Frye E, et al. A simple modi cation in operative technique can re- duce the incidence of nonanastomotic biliary strictures after orthotopic liver transplantation. Hepatology 1995;21:63-9. [Crossref] [PubMed]
- Chan EY, Olson LC, Kisthard JA, et al. Ischemic cholangiopathy following liver transplantation from donation after cardiac death donors. Liver Transpl 2008;14:604-10. [Crossref] [PubMed]
- Henrion J. Ischemia/reperfusion injury of the liver: pathophysiologic hypotheses and potential relevance to human hypoxic hepatitis. Acta Gastroenterol Belg 2000;63:336-47. [PubMed]
- Roos FJM, Poley JW, Polak WG, et al. Biliary complications after liver transplantation; recent developments in etiology, diagnosis and endoscopic treatment. Best Pract Res Clin Gastroenterol 2017;31:227-35. [Crossref] [PubMed]
- Jay CL, Lyuksemburg V, Ladner DP, et al. Ischemic cholangiopathy after controlled donation after cardiac death liver transplan- tation: a meta-analysis. Ann Surg 2011;253:259-64. [Crossref] [PubMed]
- Jay C, Ladner D, Wang E, et al. A comprehensive risk assessment of mortality following donation after cardiac death liver transplant — an analysis of the national registry. J Hepatol 2011;55:808-13. [Crossref] [PubMed]
- Reich DJ, Mulligan DC, Abt PL, et al. ASTS recommended practice guidelines for controlled donation after cardiac death organ procurement and transplantation. Am J Transplant 2009;9:2004-11. [Crossref] [PubMed]
- Taner CB, Bulatao IG, Willingham DL, et al. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl 2012;18:100-11. [Crossref] [PubMed]
- Buis CI, Verdonk RC, Van der Jagt EJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. Late presentation. Liver Transpl 2007;13:708-18. [Crossref] [PubMed]
- Kubota T, Thomson A, Clouston AD, et al. Clinicopathologic findings of recurrent primary sclerosing cholangitis after orthotopic liver transplantation. J Hepatobiliary Pancreat Surg 1999;6:377-81. [Crossref] [PubMed]
- Vera A, Moledina S, Gunson B, et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet 2002;360:1943-4. [Crossref] [PubMed]
- Clavien PA, Camargo CA, Baillie J, et al. Sphincter of Oddi dysfunction after liver transplantation. Dig Dis Sci 1995;40:73-4. [Crossref] [PubMed]
- Copelan A, Bahoura L, Tardy F, et al. Etiology, Diagnosis, and Management of Bilomas: A Current Update. Tech Vasc Interv Radiol 2015;18:236-43. [Crossref] [PubMed]
- Tung BY, Kimmey MB. Biliary Complications of Orthotopic Liver Transplantation. Dig Dis 1999;17:133-44. [Crossref] [PubMed]
- Sheng R, Ramirez CB, Zajko AB, et al. Biliary stones and sludge in liver transplant patients: a 13-year experience. Radiology 1996;198:243-7. [Crossref] [PubMed]
- Singh N, Yee Chang F, Gayowski T, et al. Fever in liver transplant recipients in the intensive care unit 1. Clin Transplant 1999;13:504-11. [Crossref] [PubMed]
- Hussaini SH, Sheridan MB, Davies M. The predictive value of transabdominal ultrasonography in the diagnosis of biliary tract complications after orthotopic liver transplantation. Gut 1999;45:900-3. [Crossref] [PubMed]
- Scharschmidt BF, Goldberg HI, Schmid R. Approach to the Patient with Cholestatic Jaundice. N Engl J Med 1983;308:1515-9. [Crossref] [PubMed]
- Harbin WP, Mueller PR, Ferrucci JT. Transhepatic cholangiography: complicatons and use patterns of the fine-needle technique: a multi-institutional survey. Radiology 1980;135:15-22. [Crossref] [PubMed]
- Laghi A, Pavone P, Catalano C, et al. MR cholangiography of late biliary complications after liver transplantation. AJR Am J Roentgenol 1999;172:1541-6. [Crossref] [PubMed]
- Fulcher AS, Turner MA. Orthotopic Liver Transplantation: Evaluation with MR Cholangiography. Radiology 1999;211:715-22. [Crossref] [PubMed]
- Boraschi P, Braccini G, Gigoni R, et al. Detection of biliary complications after orthotopic liver transplantation with MR cholangiography. Magn Reson Imaging 2001;19:1097-105. [Crossref] [PubMed]
- Kitazono MT, Qayyum A, Yeh BM, et al. Magnetic resonance cholangiography of biliary strictures after liver transplantation: A prospective double-blind study. J Magn Reson Imaging 2007;25:1168-73. [Crossref] [PubMed]
- Boraschi P, Donati F, Gigoni R, et al. MR cholangiography in orthotopic liver transplantation: sensitivity and specificity in detecting biliary complications. Clin Transplant 2010;24:E82-7. [Crossref] [PubMed]
- Meersschaut V, Mortelé KJ, Troisi R, et al. Value of MR cholangiography in the evaluation of postoperative biliary complications following orthotopic liver transplantation. Eur Radiol 2000;10:1576-81. [Crossref] [PubMed]
- Ott R, Greess H, Aichinger U, et al. Clinical value of MRC in the follow-up of liver transplant patients with a choledochojejunostomy. Abdom Imaging 2002;27:336-43. [Crossref] [PubMed]
- Boraschi P, Donati F, Gigoni R, et al. Biliary complications following orthotopic liver transplantation: May contrast-enhanced MR Cholangiography provide additional information? Eur J Radiol Open 2016;3:108-16. [Crossref] [PubMed]
- Saleem A, Baron T, Gostout C, et al. Endoscopic retrograde cholangiopancreatography using a single-balloon enteroscope in patients with altered Roux-en-Y anatomy. Endoscopy 2010;42:656-60. [Crossref] [PubMed]
- Williams EJ, Taylor S, Fairclough P, et al. Risk factors for complication following ERCP; results of a large-scale, prospective multicenter study. Endoscopy 2007;39:793-801. [Crossref] [PubMed]
- Freeman ML, Nelson DB, Sherman S, et al. Complications of Endoscopic Biliary Sphincterotomy. N Engl J Med 1996;335:909-18. [Crossref] [PubMed]
- Balderramo D, Bordas JM, Sendino O, et al. Complications after ERCP in liver transplant recipients. Gastrointest Endosc 2011;74:285-94. [Crossref] [PubMed]
- Sanna C, Giordanino C, Giono I, et al. Safety and Efficacy of Endoscopic Retrograde Cholangiopancreatography in Patients with Post-Liver Transplant Biliary Complications: Results of a Cohort Study with Long-Term Follow-Up. Gut Liver 2011;5:328-34. [Crossref] [PubMed]
- Sanna C, Saracco GM, Reggio D, et al. Endoscopic Retrograde Cholangiopancreatography in Patients With Biliary Complications After Orthotopic Liver Transplantation: Outcomes and Complications. Transplant Proc 2009;41:1319-21. [Crossref] [PubMed]
- Ambrus RB, Svendsen LB, Hillingsø JG, et al. Post-Endoscopic Retrograde Cholangiopancreaticography complications in liver transplanted patients, a single-center experience. Scand J Surg 2015;104:86-91. [Crossref] [PubMed]
- Hüsing A, Cicinnati VR, Maschmeier M, et al. Complications after endoscopic sphincterotomy in liver transplant recipients: A retrospective single-centre study. Arab J Gastroenterol 2015;16:46-9. [Crossref] [PubMed]
- Wadhawan M, Kumar A. Management issues in post living donor liver transplant biliary strictures. World J Hepatol 2016;8:461. [Crossref] [PubMed]
- Draganov P, Hoffman B, Marsh W, et al. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc 2002;55:680-6. [Crossref] [PubMed]
- Foutch PG, Sivak MV. Therapeutic endoscopic balloon dilatation of the extrahepatic biliary ducts. Am J Gastroenterol 1985;80:575-80. [PubMed]
- Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl 2006;12:88-94. [Crossref] [PubMed]
- Costamagna G, Pandolfi M, Mutignani M, et al. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc 2001;54:162-8. [Crossref] [PubMed]
- Chan CH, Donnellan F, Byrne MF, et al. Response to endoscopic therapy for biliary anastomotic strictures in deceased versus living donor liver transplantation. Hepatobiliary Pancreat Dis Int 2013;12:488-93. [Crossref] [PubMed]
- Rerknimitr R, Sherman S, Fogel EL, et al. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc 2002;55:224-31. [Crossref] [PubMed]
- Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int 2003;23:156-62. [Crossref] [PubMed]
- Morelli G, Fazel A, Judah J, et al. Rapid-sequence endoscopic management of posttransplant anastomotic biliary strictures. Gastrointest Endosc 2008;67:879-85. [Crossref] [PubMed]
- Morelli J, Mulcahy HE, Willner IR, et al. Long-Term Outcomes for Patients with Post-Liver Transplant Anastomotic Biliary Strictures Treated by Endoscopic Stent Placement. Gastrointest Endosc 2003;58:374-9. [Crossref] [PubMed]
- Poley JW, Lekkerkerker MN, Metselaar HJ, et al. Clinical outcome of progressive stenting in patients with anastomotic strictures after orthotopic liver transplantation. Endoscopy 2013;45:567-70. [Crossref] [PubMed]
- Tabibian JH, Asham EH, Han S, et al. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video). Gastrointest Endosc 2010;71:505-12. [Crossref] [PubMed]
- Alazmi WM, Fogel E, Watkins J, et al. Recurrence Rate of Anastomotic Biliary Strictures in Patients who have had Previous Successful Endoscopic Therapy for Anastomotic Narrowing after Orthotopic Liver Transplantation. Endoscopy 2006;38:571-4. [Crossref] [PubMed]
- Martins FP, Kahaleh M, Ferrari AP. Management of liver transplantation biliary stricture: Results from a tertiary hospital. World J Gastrointest Endosc 2015;7:747. [Crossref] [PubMed]
- Kaffes A, Griffin S, Vaughan R, et al. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap Adv Gastroenterol 2014;7:64-71. [Crossref] [PubMed]
- Faleschini G, Vadalà di Prampero SF, Bulajic M, et al. Predictors of endoscopic treatment outcome in the management of biliary complications after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 2015;27:150-4. [Crossref] [PubMed]
- Koksal AS, Eminler AT, Parlak E, et al. Management of biliary anastomotic strictures after liver transplantation. Transplant Rev (Orlando) 2017;31:207-17. [Crossref] [PubMed]
- Costamagna G, Boškoski I. Current treatment of benign biliary strictures. Ann Gastroenterol. 2013;26:37-40. [PubMed]
- Kahaleh M, Behm B, Clarke BW, et al. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video). Gastrointest Endosc 2008;67:446-54. [Crossref] [PubMed]
- Mahajan A, Ho H, Sauer B, et al. Temporary placement of fully covered self-expandable metal stents in benign biliary strictures: midterm evaluation (with video). Gastrointest Endosc 2009;70:303-9. [Crossref] [PubMed]
- Devière J, Reddy DN, Püspök A, et al. Successful Management of Benign Biliary Strictures With Fully Covered Self-Expanding Metal Stents. Gastroenterology 2014;147:385-95. [Crossref] [PubMed]
- Kahaleh M, Brijbassie A, Sethi A, et al. Multicenter Trial Evaluating the Use of Covered Self-expanding Metal Stents in Benign Biliary Strictures. J Clin Gastroenterol 2013;47:695-9. [Crossref] [PubMed]
- Cerecedo-Rodriguez J, Phillips M, Figueroa-Barojas P, et al. Self Expandable Metal Stents for Anastomotic Stricture Following Liver Transplant. Dig Dis Sci 2013;58:2661-6. [Crossref] [PubMed]
- Hu B, Gao D, Yu F, et al. Endoscopic stenting for post-transplant biliary stricture: usefulness of a novel removable covered metal stent. J Hepatobiliary Pancreat Sci 2011;18:640-5. [Crossref] [PubMed]
- Haapamäki C, Udd M, Halttunen J, et al. Endoscopic treatment of anastomotic biliary complications after liver transplantation using removable, covered, self-expandable metallic stents. Scand J Gastroenterol 2012;47:116-21. [Crossref] [PubMed]
- Vandenbroucke F, Plasse M, Dagenais M, et al. Treatment of post liver transplantation bile duct stricture with self-expandable metallic stent. HPB (Oxford) 2006;8:202-5. [Crossref] [PubMed]
- Poley JW, Cahen DL, Metselaar HJ, et al. A prospective group sequential study evaluating a new type of fully covered self-expandable metal stent for the treatment of benign biliary strictures (with video). Gastrointest Endosc 2012;75:783-9. [Crossref] [PubMed]
- Tarantino I, Barresi L, Curcio G, et al. Definitive outcomes of self-expandable metal stents in patients with refractory post-transplant biliary anastomotic stenosis. Dig Liver Dis 2015;47:562-5. [Crossref] [PubMed]
- Traina M, Tarantino I, Barresi L, et al. Efficacy and safety of fully covered self-expandable metallic stents in biliary complications after liver transplantation: A preliminary study. Liver Transpl 2009;15:1493-8. [Crossref] [PubMed]
- García-Pajares F, Sánchez-Antolín G, Pelayo SL, et al. Covered Metal Stents for the Treatment of Biliary Complications after Orthotopic Liver Transplantation. Transplant Proc 2010;42:2966-9. [Crossref] [PubMed]
- Sauer P, Chahoud F, Gotthardt D, et al. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy 2012;44:536-8. [Crossref] [PubMed]
- Tarantino I, Traina M, Mocciaro F, et al. Fully covered metallic stents in biliary stenosis after orthotopic liver transplantation. Endoscopy 2012;44:246-50. [Crossref] [PubMed]
- Chaput U, Scatton O, Bichard P, et al. Temporary placement of partially covered self-expandable metal stents for anastomotic biliary strictures after liver transplantation: a prospective, multicenter study. Gastrointest Endosc 2010;72:1167-74. [Crossref] [PubMed]
- Khan MA, Baron TH, Kamal F, et al. Efficacy of self-expandable metal stents in management of benign biliary strictures and comparison with multiple plastic stents: a meta-analysis. Endoscopy 2017;49:682-94. [Crossref] [PubMed]
- Costamagna G. Efficacy of self-expandable metal stents compared with multiple plastic stents in benign biliary strictures. Endoscopy 2018;50:648. [Crossref] [PubMed]
- Landi F, de’Angelis N, Sepulveda A, et al. Endoscopic treatment of anastomotic biliary stricture after adult deceased donor liver transplantation with multiple plastic stents versus self-expandable metal stents: a systematic review and meta-analysis. Transpl Int 2018;31:131-51. [Crossref] [PubMed]
- Ng S, Tan KAL, Anil G. The role of interventional radiology in complications associated with liver transplantation. Clin Radiol 2015;70:1323-35. [Crossref] [PubMed]
- Li Y, Sun H, Yan X, et al. Magnetic compression anastomosis for the treatment of benign biliary strictures: a clinical study from China. Surg Endosc 2020;34:2541-50. [Crossref] [PubMed]
- Porayko MK, Kondo M, Steers JL. Liver transplantation: late complications of the biliary tract and their management. Semin Liver Dis 1995;15:139-55. [Crossref] [PubMed]
- Rizk RS, McVicar JP, Emond MJ, et al. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc 1998;47:128-35. [Crossref] [PubMed]
- Verdonk RC, Buis CI, van der Jagt EJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl 2007;13:725-32. [Crossref] [PubMed]
- Llach J, Bordas JM, Elizalde JI, et al. Sphincterotomy in the treatment of biliary leakage. Hepatogastroenterology 2002;49:1496-8. [PubMed]
- Thuluvath PJ, Pfau PR, Kimmey MB, et al. Biliary Complications after Liver Transplantation: the Role of Endoscopy. Endoscopy 2005;37:857-63. [Crossref] [PubMed]
- Gilsdorf JR, Phillips M, McLeod MK, et al. Radionuclide evaluation of bile leakage and the use of subhepatic drains after cholecystectomy. Am J Surg 1986;151:259-62. [Crossref] [PubMed]
- Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol 2008;14:493-7. [Crossref] [PubMed]
- Cotton PB, Durkalski V, Romagnuolo J, et al. Effect of Endoscopic Sphincterotomy for Suspected Sphincter of Oddi Dysfunction on Pain-Related Disability Following Cholecystectomy. JAMA 2014;311:2101. [Crossref] [PubMed]
- Cotton PB, Pauls Q, Keith J, et al. The EPISOD study: long-term outcomes. Gastrointest Endosc 2018;87:205-10. [Crossref] [PubMed]
- Shah JN, Haigh WG, Lee SP, et al. Biliary casts after orthotopic liver transplantation: clinical factors, treatment, biochemical analysis. Am J Gastroenterol 2003;98:1861-7. [Crossref] [PubMed]
- Del Bello A, Congy-Jolivet N, Danjoux M, et al. Donor-specific antibodies and liver transplantation. Hum Immunol 2016;77:1063-70. [Crossref] [PubMed]
- den Dulk AC, Shi X, Verhoeven CJ, et al. Donor-specific anti-HLA antibodies are not associated with nonanastomotic biliary strictures but both are independent risk factors for graft loss after liver transplantation. Clin Transplant 2018;32:e13163. [Crossref] [PubMed]
- Weeder PD, van Rijn R, Porte RJ, et al. Machine perfusion in liver transplantation as a tool to prevent non-anastomotic biliary strictures: Rationale, current evidence and future directions. J Hepatol 2015;63:265-75. [Crossref] [PubMed]
- Karimian N, Westerkamp AC, Porte RJ. Biliary complications after orthotopic liver transplantation. Curr Opin Organ Transplant 2014;19:209-16. [Crossref] [PubMed]
- Singhal A, Wima K, Hoehn RS, et al. Hospital Resource Use with Donation after Cardiac Death Allografts in Liver Transplantation: A Matched Controlled Analysis from 2007 to 2011. J Am Coll Surg 2015;220:951-8. [Crossref] [PubMed]
- Ceresa CDL, Nasralla D, Coussios CC, et al. The case for normothermic machine perfusion in liver transplantation. Liver Transpl 2018;24:269-75. [Crossref] [PubMed]
- Seehofer D, Eurich D, Veltzke-Schlieker W, et al. Biliary Complications After Liver Transplantation: Old Problems and New Challenges. Am J Transplant 2013;13:253-65. [Crossref] [PubMed]
- Ravikumar R, Jassem W, Mergental H, et al. Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transplant 2016;16:1779-87. [Crossref] [PubMed]
- Robertson FP, Bessell PR, Diaz-Nieto R, et al. High serum Aspartate transaminase levels on day 3 postliver transplantation correlates with graft and patient survival and would be a valid surrogate for outcome in liver transplantation clinical trials. Transpl Int 2016;29:323-30. [Crossref] [PubMed]
- Quillin RC, Guarrera JV. Hypothermic machine perfusion in liver transplantation. Liver Transpl 2018;24:276-81. [Crossref] [PubMed]
- Guarrera JV, Henry SD, Samstein B, et al. Hypothermic Machine Preservation in Human Liver Transplantation: The First Clinical Series. Am J Transplant 2010;10:372-81. [Crossref] [PubMed]
- Dutkowski P, Polak WG, Muiesan P, et al. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann Surg 2015;262:764-70; discussion 770-1. [Crossref] [PubMed]
- Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation facilitates successful transplantation of "orphan" extended criteria donor livers. Am J Transplant 2015;15:161-9. [Crossref] [PubMed]
- Schlegel A, Muller X, Kalisvaart M, et al. Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol 2019;70:50-7. [Crossref] [PubMed]
- Boteon YL, Boteon AP, Attard J, et al. Impact of machine perfusion of the liver on post-transplant biliary complications: A systematic review. World J Transplant 2018;8:220-31. [Crossref] [PubMed]
- Koneru B, Dikdan G. Hepatic steatosis and liver transplantation current clinical and experimental perspectives. Transplantation 2002;73:325-30. [Crossref] [PubMed]
- Annual report on liver transplantation. 2006; Available online: https://nhsbtdbe.blob.core.windows.net/umbraco-assets-corp/1314/organ_specific_report_liver_2016.pdf
- Nagrath D, Xu H, Tanimura Y, et al. Metabolic preconditioning of donor organs: Defatting fatty livers by normothermic perfusion ex vivo. Metab Eng 2009;11:274-83. [Crossref] [PubMed]
- Boteon YL, Boteon APCS, Attard J, et al. Ex situ machine perfusion as a tool to recondition steatotic donor livers: Troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am J Transplant 2018;18:2384-99. [Crossref] [PubMed]
- Laukkarinen J, Nordback I, Mikkonen J, et al. A novel biodegradable biliary stent in the endoscopic treatment of cystic-duct leakage after cholecystectomy. Gastrointest Endosc 2007;65:1063-8. [Crossref] [PubMed]
- Xu X, Liu T, Liu S, et al. Feasibility of biodegradable PLGA common bile duct stents: An in vitro and in vivo study. J Mater Sci Mater Med 2009;20:1167-73. [Crossref] [PubMed]
- Janousek L, Maly S, Oliverius M, et al. Bile Duct Anastomosis Supplied With Biodegradable Stent in Liver Transplantation: The Initial Experience. Transplant Proc 2016;48:3312-6. [Crossref] [PubMed]





