
Assessing resectability for colorectal liver metastases: agreeing that we disagree
Colorectal cancer (CRC) is the second leading cause of cancer related mortality, though improvements in CRC screening, systemic and local treatment, and surgical technique have steadily won victories in the battle against CRC, and the rate of mortality from CRC is steadily declining (1). However, perhaps no clinical-oncologic scenario is as unstandardized as the management of colorectal-liver metastases (CRLM) and their evaluation for consideration of resectability. Previous work has demonstrated the low concordance between medical oncologists and cancer subspecialists in this regard (2,3), but perhaps more striking is the inability for surgeons to arrive to consensus on what constitutes resectable or unresectable anatomy (4). Not surprisingly, methodologically robust studies have demonstrated variations in surgical resection practices. Fenton et al. recently published an exhaustive analysis of 7 years of data from the CORECT-R repository, encompassing 7,423 hepatic resections across the NHS system in England (5). Here we will highlight key findings from this report, and explore potential drivers of the observed variability in the care of patients with CRLM.
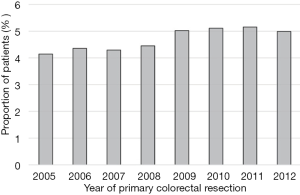
Noteworthy, findings from Fenton et al. include the report of steady increases in the number of hepatectomies conducted for CRLM (Figure 1), plateauing at around 5% of patients. However, despite the increased rate of resection, the number of major liver resections declined over this period of time (48.2% vs. 39.9%, P<0.001), perhaps due to closer adherence to parenchymal sparing principles (5). However, patients were more likely to be offered surgical resection for CRLM if their primary resection occurred at centers with dedicated hepato-pancreato-biliary (HPB) teams, suggesting a difference in referral pathways between hospitals. Furthermore, patients who underwent liver resection at a center without a dedicated liver specialist team were more likely to undergo major liver resections (41.1 vs. 36.1, P<0.001).
Over the last two decades advances in liver resection techniques such as vascular modulation to stimulate hyperplasia and hypertrophy (6), two stage hepatectomy including associating liver partition with portal vein ligation (7), and exploratory protocols for liver transplantation for CRLM (8) have bucked the status quo of what is resectable liver anatomy. Indeed, the observed pattern of discordance in patient care reported by Fenton et al. is not reserved to the centralized system of the NHS. A recent report by Raoof et al. found similar disparities in access to hepatic resection for CRLM based on both the medical service area and the neighborhood-area in which a patient resides in (9) (Figure 2). This is not a trivial finding as lack of access to hepatic resection was tied to worse survival in the underserved regions. Yet, despite consensus statements in the approach to patients with CRLM (10), agreement between expert HPB surgeons, the cadres of whom may train through various fellowships en route to becoming HPB experts, is lacking. This can result in confusion for referring practitioners, and highlights the need to educate referring physicians such as medical oncologist and general surgeons to encourage seeking multiple opinions. Recent concomitant advances in the care of patients with CRLM may explain the multiplicity of approaches employed between center, and in the future we may reach an equilibrium of established protocols, not unlike the assessment of resectability for pancreatic cancer in the head of the pancreas, however communally we must push for establishing these pathways to optimize the care of these patients.

Beyond the discordance in resection rate based on hospital location and access to HPB teams, Fenton et al. also uncovered patient characteristics which correlated with lower observed rates of resection. For example, right sided primary lesions, advanced age, additional comorbidities, socioeconomic deprivation, and female gender were all found to associate with fewer hepatic resections. While some of these variables, such as right sided CRCs, may portend a more aggressive tumor biology, and thus obviate the opportunity for liver resection in upon stage IV disease (11), the finding that women receive less hepatectomies for the same disease should be highlighted, and efforts to understand the biological, social, and cognitive factors which result in this discrepancy explored. Similarly, efforts to understand the access to liver surgery for patients >70 years of age, and those residing within an economically deprived household will necessitate multidisciplinary interventions to bridge the divide in care.
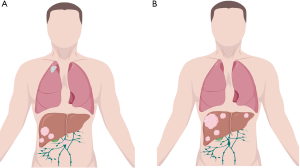
From the 10,000-foot view offered by Fenton et al. we can see that the advance against CRLM continues despite the interceding challenges presented by a complex surgical procedure. Ultimately, stakeholders including physicians, patients, payers, and administrators will be required to streamline timely referral and adequate multidisciplinary evaluation for patients presenting with CRLM. However, as we train the next generation of HPB surgeons, we must ensure that our trainees gain exposure to a multiplicity of approaches in the management of these patients (12), and become versed in quality and outcome science, so that a prevailing consensus and standardization of care may develop. We must entrain the next generation of HPB surgeons to assess resectability from both a technical and oncologic perspective. Take for example the case of patient with right sided hepatic metastases and a 70% future liver remnant, with concomitant periportal nodes which are PET avid and solitary pulmonary metastases (Figure 3A) and the case of a patient with bilobar metastatic disease and a 28% future liver remnant, with no evidence of extrahepatic disease and biochemical response to FOLFOX-Bevacizumab (Figure 3B). The first patient, while technically resectable from an HPB perspective is likely to garner little oncologic benefit in the long run; conversely the second patient may present a borderline technically resectable patient, but could achieve long term disease control with the appropriate surgical plan.
Given the heterogeneity between HPB surgeons and loco-regional practices, patients and referring physicians should view surgical opinions of resectability for hepatic colorectal liver metastases with a grain of salt, and seek second and third opinions from multiple HPB surgeons prior to closing the door at a potential curative intervention.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office of Hepatobiliary Surgery and Nutrition. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.25/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Aubin JM, Bressan AK, Grondin SC, et al. Assessing resectability of colorectal liver metastases: How do different subspecialties interpret the same data? Can J Surg 2018;61:251-6. [Crossref] [PubMed]
- Choti MA, Thomas M, Wong SL, et al. Surgical Resection Preferences and Perceptions among Medical Oncologists Treating Liver Metastases from Colorectal Cancer. Ann Surg Oncol 2016;23:375-81. [Crossref] [PubMed]
- Mohammad WM, Martel G, Mimeault R, et al. Evaluating agreement regarding the resectability of colorectal liver metastases: a national case-based survey of hepatic surgeons. HPB (Oxford) 2012;14:291-7. [Crossref] [PubMed]
- Fenton HM, Taylor JC, Lodge JPA, et al. Variation in the Use of Resection for Colorectal Cancer Liver Metastases. Ann Surg 2019;270:892-8. [Crossref] [PubMed]
- Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg 2001;88:165-75. [Crossref] [PubMed]
- Pineda-Solís K, Paskar D, Tun-Abraham M, et al. Expanding the limits of resectability: Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) using monosegment 6, facilitated by an inferior right hepatic vein. J Surg Oncol 2017;115:959-62. [Crossref] [PubMed]
- Dueland S, Syversveen T, Solheim JM, et al. Survival Following Liver Transplantation for Patients With Nonresectable Liver-only Colorectal Metastases. Ann Surg 2020;271:212-8. [Crossref] [PubMed]
- Raoof M, Haye S, Ituarte PHG, et al. Liver Resection Improves Survival in Colorectal Cancer Patients: Causal-effects From Population-level Instrumental Variable Analysis. Ann Surg 2019;270:692-700. [Crossref] [PubMed]
- Adams RB, Aloia TA, Loyer E, et al. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB (Oxford) 2013;15:91-103. [Crossref] [PubMed]
- Missiaglia E, Jacobs B, D'Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995-2001. [Crossref] [PubMed]
- D'Angelica MI, Chapman WC. HPB Surgery: The Specialty is Here to Stay, but the Training is in Evolution. Ann Surg Oncol 2016;23:2123-5. [Crossref] [PubMed]


