
Stereotactic body radiation therapy versus radiofrequency ablation in patients with small hepatocellular carcinoma: a systematic review and meta-analysis
Introduction
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer-related deaths worldwide (1,2). The major treatments for HCC include surgical intervention (cancer resection or liver transplantation) (3), systemic chemotherapy [such as oxaliplatin-based chemotherapy (OXA), infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX), gemcitabine plus oxaliplatin (GEMOX)] (4,5), targeted therapy [such as sorafenib and lenvatinib] (6,7), local therapy [transhepatic arterial chemoembolization (TACE)] (8), immunotherapy [programmed cell death protein 1 (PD-1)/programmed death-ligand 1 (PD-L1) ] (7), percutaneous ablation [such as radiofrequency ablation (RFA)], and radiotherapy [such as conventional external beam radiation and stereotactic body radiation therapy (SBRT)] (9). A quantitative analysis is necessary to evaluate the efficiency of various treatment options.
RFA is a first-line treatment for HCC and is as effective as surgical resection when the tumor is smaller than 3 cm in size (10). RFA has shown favourable local control and survival in small, non-resectable HCC patients (11,12). Currently, novel RFA technique [No-touch multipolar radiofrequency ablation (NTM-RFA), percutaneous RFA, etc.] are available for the treatment of medium-size HCC (2–5 cm) (13-15).
Although HCC is known as a radiosensitive tumor, the use of radiotherapy is limited because of the poor radiation tolerance and complexity of tumor localization (16). However, advances in modern treatment design and delivery have reestablished the interest in radiotherapy as an effective locoregional treatment. Modern radiotherapy techniques have allowed clinicians to increase the dose while sparing normal liver function, thus avoiding radiation-induced liver disease (RILD) (16). RILD was defined as the presence of anicteric ascites with at least a two-fold increase in the alkaline phosphatase level compared to the pretreatment level in the absence of progression or at least a five-fold increase in the transaminase levels above the normal upper limit or pretreatment level within 3 months of SBRT or a decline in liver function (measured by a deterioration in the Child-Pugh score by 2 or more) (17,18). SBRT is a radioactive, locally ablative treatment that is an alternative to HCC monotherapy. Moreover, it serves as a bridge for patients who qualify for transplantation or other multimodality treatments (19). It has a high precision for small mass and a small number of tumors in liver (16,20). SBRT might be a reasonably better first-line treatment regime for inoperable HCC than RFA (21). Here, we performed a systematic review and meta-analysis of published literature to compare the clinical outcomes between SBRT and RFA in HCC patients. We present the following article in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting checklist (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.15/rc) (22).
Methods
Study design
We developed a protocol that defined the inclusion criteria, search strategy, outcomes of interest, and analysis plan.
Procedures
To collect studies for our systematic review and meta-analysis, we searched the electronic databases including Embase, PubMed, and the Cochrane Central Register of Controlled Trials (CENTRAL) from the date of inception of every database to September 2019. The search terms are provided in Supplementary File.
We used the following inclusion criteria for determining the eligibility of the study populations (hereafter referred to as cohorts) (Figure 1):
- Patients with HCC (size ≤5 cm) or residual HCC (RHCC, size ≤5 cm);
- Treatment with only SBRT or RFA;
- Reported relevant outcomes such as overall survival (OS), freedom from local progression (FFLP), and complications;
- From an original study [randomized controlled trial (RCT), non-randomized clinical trial, observational studies, cohort studies, and retrospective studies].
We did not restrict our search to language, country, patients’ characteristics, or underlying disease status (primary or recurrent). We excluded case reports, reviews, notes, letters, errata, commentaries, studies published only as abstracts, and studies with a clear duplication.
We extracted the following data: author, study period, patients’ characteristics (i.e., sex, age, tumor size, tumor number, and Child-Pugh class), the number of patients and their characteristics after propensity score matching (PSM), interventions (radiation dose and fractionation schedule), length of follow-up, and relevant outcomes. We defined relevant outcomes as OS at 1 and 2 years, FFLP, toxic effects, and functional status. We defined relevant toxicity as Child-Pugh class degeneration, liver failure, and RILD.
Statistical analysis
We analyzed the patients who received SBRT or RFA after PSM. We calculated event rates of the outcome (the proportion of patients who developed relevant outcomes) from the included cohorts for those two therapies. We pooled log-transformed event rates and assessed heterogeneity using the Mantel-Haenszel test (16,23). A statistical test with a P value <0.05 was considered significant. To account for the potential effect of publication bias, the methodological quality of literature was assessed by the risk of bias table from Cochrane Collaboration. To measure overall heterogeneity across the included cohorts, we calculated the I2 statistic, with I2 >50% indicating high heterogeneity. We performed the statistical analyses in RevMan 5.3 software (RevMan Web, Cochrane Collaboration, USA).
Results
Out of 291 studies that were collected from the database search, 5 retrospective studies met the inclusion criteria and were included in this systematic review (17,21,24-26) (Figure 1). We did not find any RCTs or controlled studies that directly compared SBRT with RFA. From these 5 studies, 5 cohorts were identified and 620 patients were treated with SBRT whereas 4194 patients received RFA (17,21,24-26) (Table 1). The study by Rajyaguru et al. confirmed that the patients who received SBRT were older than those who received RFA (≥71 years, P<0.001) (24), but the other four studies found no significant difference in median age (17,21,25,26). The proportions of tumor larger than 3cm are 38.4% and 31.9% in SBRT and RFA group accordingly (P=0.004). The median tumor size and proportion of men did not significantly differ between the two groups. The study by Rajyaguru et al. did not offer any specific data on the Child-Pugh class of the patients. However, the four other studies reported that patients who received SBRT had better Child-Pugh class than those who received RFA (Table 1).
Table 1
| Characteristic | Cohorts | SBRT | RFA | P value |
|---|---|---|---|---|
| Patients (n) | 5 | 620 | 4194 | – |
| Men (%) | 5 | 74.0 | 72.2 | 0.353 |
| Median age (year)1 | 4 | 66.9 [35–93] | 67.0 [31–90] | – |
| Median tumor size (cm) | 5 | 2.4 | 2.4 | 0.563 |
| Tumor number (/patients) | 3 | 418/311 | 1,459/1,060 | 0.379 |
| Median Child-Pugh class, n (%) | 4 | |||
| A | 307 (89.2) | 448 (75.6) | <0.001 | |
| B | 35 (10.2) | 124 (20.9) | <0.001 | |
| C | 2 (0.6) | 21 (3.5) | 0.005 |
1, the information in Rajyaguru
The methodological quality of the included studies was fair (Figure 2). All studies provided adequate outcome ascertainment, enrolled a representative sample of patients, and had an acceptable length of follow-up. However, because of the differences between the two treatment strategies, performance bias cannot be avoided. Assessment of publication bias was not done because data would be unreliable because of the small number of studies included in each treatment group. The included studies had high heterogeneity and I2 >50%.
As shown in Table 2, the pooled event rate of OS at 1 year in the SBRT group and RFA group showed no significant difference (P=0.14). However, the pooled event rate of OS at 2 years in SBRT was significantly lower than that in RFA [Figure 3; odds ratio (OR): 0.63, P=0.0001]. The data involved in OS analysis showed no heterogeneity (I2 =0%). Interestingly, the pooled event rate of FFLP at 2 years in SBRT was higher than that in RFA (Figure 4, odds ratio 1.66, P=0.03). Moreover, the data involved in the FFLP analysis showed no heterogeneity (I2 =0%).
Table 2
| Group | Cohorts | Patients (n) | Event (%) | P value |
|---|---|---|---|---|
| 1-year OS | 0.140 | |||
| SBRT | 4 | 287 | 84.7 | |
| RFA | 4 | 385 | 80.3 | |
| 2-year OS | 0.0001 | |||
| SBRT | 5 | 562 | 49.6 | |
| RFA | 5 | 906 | 56.7 | |
| 2-year FFLP | 0.03 | |||
| SBRT | 3 | 181 | 75.7 | |
| RFA | 3 | 279 | 70.6 |
OS, overall survival; FFLP, freedom from local progression; SBRT, stereotactic body radiation therapy; RFA, radiofrequency ablation.
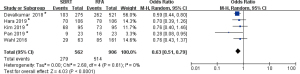
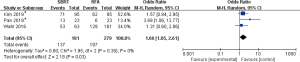
Table 3 shows the major severe liver complications in RFA or grade 3/4 adverse effects in SBRT. The deterioration of the Child-Pugh class showed no significant difference between the two groups (P=0.23). The patients who received SBRT were more likely to develop liver dysfunction than those who received RFA (P=0.033). In addition, RILD was reported in two studies (17,21). Kim et al. reported that seven patients (6.7%) developed RILD in the SBRT group including one patient had an elevated transaminase levels five times the normal upper limit, four patients had elevated alkaline phosphatase levels at twice the pretreatment levels, and two patients with Child-Pugh class A, and these patients further deteriorated to Child-Pugh class B or C (17). Only one patient with RILD was reported by Wahl et al. (21).
Table 3
| Variable | Cohorts | Events | Total | Events rate (%) | P value |
|---|---|---|---|---|---|
| Child-Pugh degeneration | |||||
| SBRT | 1 | 9 | 113 | 8.0 | 0.23 |
| RFA | 1 | 24 | 231 | 10.4 | |
| Liver failure | |||||
| SBRT | 2 | 4 | 136 | 2.9 | 0.033 |
| RFA | 2 | 1 | 254 | 0.4 |
SBRT, stereotactic body radiation therapy; RFA, radiofrequency ablation.
Discussion
A total of 5 studies met the inclusion criteria and were included in this meta-analysis. Due to the limitations of different researches and diverse description standards adopted in the included studies, we could not compare the OS or FFLP situation at 3 or 5 years completely, thus 2-year FFIP and OS became a better research indicator. Based on pooled results, we found that SBRT has a better effect on the control of local tumor recurrence than RFA. On the other hand, patients treated with RFA have a better survival rate than SBRT. This suggests that the radiation damage caused by SBRT and the heterogeneity of the constitutions of the included patients may affect the prognosis in the SBRT group. In addition, liver toxicity was not significantly different between SBRT and RFA. We have a treatment with RFA reliable post-therapy evaluation and a treatment with SBRT-related complex evaluation. However, we could not compare other toxicities because grade 3 adverse effects directly according to the RTOG/EORTC criteria were not reported by the included studies.
Previously, several reviews have addressed the effectiveness and safety of SBRT (23,27) and RFA (10,28) separately. Although no meta-analysis of SBRT and RFA in HCC was published, Wahl et al. (21) directly compared the effectiveness of SBRT with RFA. SBRT was more effective than RFA for tumors ≥2 cm, but there was no significant difference in effectiveness between SBRT and RFA for tumors <2 cm (21). In prior studies, SBRT was used to mostly treat cases with Child-Pugh stage A or B and small target volumes which showed favorable efficacy and low toxicity for small HCC and early-stage patients. Additionally, our analysis confirmed that SBRT had a better overall local control ratio than RFA. Furthermore, similar to RFA, increasing the tumor diameter may reduce the local control ratio and OS of SBRT (29,30). However, the safety of SBRT compared to RFA needed further assessment. Previous studies indicated that SBRT monotherapy of small HCC had low toxicity (30), and the early and late toxicities overall grade from 1 to 4 occurred at very low levels (31). Our analysis reveals that the safety profile and administration of radiological dose in SBRT needs further improvement.
The dosage and grade of SBRT therapy for HCC are under study. Each study included in the analysis used a different total dose of radiation ranging from 30 to 60 Gy. Moreover, there was no consensus regarding the number of fractions used in the SBRT group. The total dose administered depends not only on the hepatic function based on the Child-Pugh class but also on the restrictions of the dose delivered to the healthy liver tissue and the dose delivered to the other organs at risk (i.e., stomach, bowels, and spinal cord) (30). Studies in which patients were administered low radiation dose may report the same efficacy as those in which patients were administered with high dose (32). Therefore, further studies should be conducted to confirm the suitable dose of SBRT for HCC.
Current clinical evidence of HCC suggests that the FFLP rate of SBRT is significantly higher than that of RFA. Moreover, SBRT has a lower long-term survival rate than RFA. It must be pointed out that the overall quantity and quality of data on SBRT and RFA are poor and may have a risk of potential bias. The effects of these two treatments should be considered comprehensively in the treatment of HCC, especially of patients with Child-Pugh stage A or B. Additionally, RCTs or large, controlled, prospective trials should be designed for further comparison of those two therapies (27,33). There is a need for improved reporting and prospective studies to unequivocally recommend SBRT as a definitive treatment option for HCC in the guidelines (30). Moreover, clarity regarding the standard radiological dose and adverse effects of RTOG/EORTC criteria is necessary for acute and late toxicity comparison when conducting prospective studies.
The application of formal meta-analytic methods to retrospective studies is controversial (16,34), and this is a major limitation of our study. The study design and population were diverse, and these differences may affect general estimates. When RCTs are not available, a meta-analysis of retrospective studies is one of the methods to evaluate the efficacy and effectiveness of SBRT (35). However, potential bias such as selection bias is inevitable due to the lack of RCTs. In addition, performance bias is inevitable due to the inherent differences between the two treatment strategies in this meta-analysis (Figure 2). The small number of studies included may further amplify the potential bias.
This study demonstrates that SBRT has better efficacy for local progression but poorer OS than RFA. Therefore, SBRT should be cautiously selected for treatment following a comprehensive analysis of patients with inoperable small HCC including the patients’ general condition, Child-Pugh score, stage of Barcelona Clinic Liver Cancer, tumor number, tumor location, prospective survival time, tolerance to radiotherapy, etc. In fact, RFA is still the preferred treatment in many cases. But SBRT appears to be an effective alternative treatment when RFA is not feasible due to the patients’ situation (17).
There were differences in the characteristics of the experimental subjects in the studies. Moreover, there were differences in the experimental methods of the included studies since there was no uniform standard for describing patient characteristics, SBRT radiological dose, and complications. Therefore, the conclusions obtained were biased inevitably. Further RCTs comparing SBRT and RFA should quantify the factors that affect tumor control and prognosis.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China under grant no. 81570591 and the Science and Technology Department of Zhejiang Province under grant no. LGF19H030017.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.15/rc
Data Sharing Statement: Available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.15/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.org/article/view/10.21037/hbsn.2020.03.15/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hartke J, Johnson M, Ghabril M. The diagnosis and treatment of hepatocellular carcinoma. Semin Diagn Pathol 2017;34:153-9. [Crossref] [PubMed]
- Costentin C. Hepatocellular carcinoma surveillance. Presse Med 2017;46:381-5. [Crossref] [PubMed]
- Kow AWC. Transplantation versus liver resection in patients with hepatocellular carcinoma. Transl Gastroenterol Hepatol 2019;4:33. [Crossref] [PubMed]
- Petrelli F, Coinu A, Borgonovo K, et al. Oxaliplatin-based chemotherapy: a new option in advanced hepatocellular carcinoma. a systematic review and pooled analysis. Clin Oncol (R Coll Radiol) 2014;26:488-96. [Crossref] [PubMed]
- Ray EM, Sanoff HK. Optimal therapy for patients with hepatocellular carcinoma and resistance or intolerance to sorafenib: challenges and solutions. J Hepatocell Carcinoma 2017;4:131-8. [Crossref] [PubMed]
- Saeki I, Yamasaki T, Maeda M, et al. Treatment strategies for advanced hepatocellular carcinoma: Sorafenib vs hepatic arterial infusion chemotherapy. World J Hepatol 2018;10:571-84. [Crossref] [PubMed]
- Alqahtani A, Khan Z, Alloghbi A, et al. Hepatocellular Carcinoma: Molecular Mechanisms and Targeted Therapies. Medicina (Kaunas) 2019;55: [Crossref] [PubMed]
- Wei Y, Liu J, Yan M, et al. Effectiveness and Safety of Combination Therapy of Transarterial Chemoembolization and Apatinib for Unresectable Hepatocellular Carcinoma in the Chinese Population: A Meta-Analysis. Chemotherapy 2019;64:94-104. [Crossref] [PubMed]
- Chen CP. Role of Radiotherapy in the Treatment of Hepatocellular Carcinoma. J Clin Transl Hepatol 2019;7:183-90. [Crossref] [PubMed]
- Xu Z, Xie H, Zhou L, et al. The Combination Strategy of Transarterial Chemoembolization and Radiofrequency Ablation or Microwave Ablation against Hepatocellular Carcinoma. Anal Cell Pathol (Amst) 2019;2019:8619096 [Crossref] [PubMed]
- Feng K, Yan J, Li X, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 2012;57:794-802. [Crossref] [PubMed]
- Vietti Violi N, Duran R, Guiu B, et al. Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 2018;3:317-25. [Crossref] [PubMed]
- Mohkam K, Dumont PN, Manichon AF, et al. No-touch multibipolar radiofrequency ablation vs. surgical resection for solitary hepatocellular carcinoma ranging from 2 to 5cm. J Hepatol 2018;68:1172-80. [Crossref] [PubMed]
- Tian G, Yang S, Yuan J, et al. Comparative efficacy of treatment strategies for hepatocellular carcinoma: systematic review and network meta-analysis. BMJ Open 2018;8:e021269 [Crossref] [PubMed]
- Nault JC, Sutter O, Nahon P, et al. Percutaneous treatment of hepatocellular carcinoma: State of the art and innovations. J Hepatol 2018;68:783-97. [Crossref] [PubMed]
- Qi WX, Fu S, Zhang Q, et al. Charged particle therapy versus photon therapy for patients with hepatocellular carcinoma: a systematic review and meta-analysis. Radiother Oncol 2015;114:289-95. [Crossref] [PubMed]
- Kim N, Kim HJ, Won JY, et al. Retrospective analysis of stereotactic body radiation therapy efficacy over radiofrequency ablation for hepatocellular carcinoma. Radiother Oncol 2019;131:81-7. [Crossref] [PubMed]
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76:S94-100. [Crossref] [PubMed]
- Gerum S, Heinz C, Belka C, et al. Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol 2018;13:100. [Crossref] [PubMed]
- Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2010;76:326-32. [Crossref] [PubMed]
- Wahl DR, Stenmark MH, Tao Y, et al. Outcomes After Stereotactic Body Radiotherapy or Radiofrequency Ablation for Hepatocellular Carcinoma. J Clin Oncol 2016;34:452-9. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Sterzing F, Brunner TB, Ernst I, et al. Stereotactic body radiotherapy for liver tumors: principles and practical guidelines of the DEGRO Working Group on Stereotactic Radiotherapy. Strahlenther Onkol 2014;190:872-81. [Crossref] [PubMed]
- Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol 2018;36:600-8. [Crossref] [PubMed]
- Hara K, Takeda A, Tsurugai Y, et al. Radiotherapy for Hepatocellular Carcinoma Results in Comparable Survival to Radiofrequency Ablation: A Propensity Score Analysis. Hepatology 2019;69:2533-45. [Crossref] [PubMed]
- Pan YX, Xi M, Fu YZ, et al. Stereotactic Body Radiotherapy as a Salvage Therapy after Incomplete Radiofrequency Ablation for Hepatocellular Carcinoma: A Retrospective Propensity Score Matching Study. Cancers (Basel) 2019;11: [Crossref] [PubMed]
- Riou O, Valdenaire S, Debuire P, et al. Liver stereotactic body radiotherapy: Clinical features and technical consequences, results. Which treatment machine in which situation? Cancer Radiother 2019;23:636-50. [Crossref] [PubMed]
- Cho E, Cho HA, Jun CH, et al. A Review of Hepatocellular Carcinoma in Elderly Patients Focused on Management and Outcomes. In Vivo 2019;33:1411-20. [Crossref] [PubMed]
- Rim CH, Kim CY, Yang DS, et al. Comparison of radiation therapy modalities for hepatocellular carcinoma with portal vein thrombosis: A meta-analysis and systematic review. Radiother Oncol 2018;129:112-22. [Crossref] [PubMed]
- Dobrzycka M, Spychalski P, Rostkowska O, et al. Stereotactic body radiation therapy for early-stage hepatocellular carcinoma - a systematic review on outcome. Acta Oncol 2019;58:1706-13. [Crossref] [PubMed]
- Zhou Y, Zhao Y, Li B, et al. Meta-analysis of radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma. BMC Gastroenterol 2010;10:78. [Crossref] [PubMed]
- Kim JW, Seong J, Lee IJ, et al. Phase I dose escalation study of helical intensity-modulated radiotherapy-based stereotactic body radiotherapy for hepatocellular carcinoma. Oncotarget 2016;7:40756-66. [Crossref] [PubMed]
- Soni PD, Palta M. Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma: Current State and Future Opportunities. Dig Dis Sci 2019;64:1008-15. [Crossref] [PubMed]
- Blettner M, Sauerbrei W, Schlehofer B, et al. Traditional reviews, meta-analyses and pooled analyses in epidemiology. Int J Epidemiol 1999;28:1-9. [Crossref] [PubMed]
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008-12. [Crossref] [PubMed]




