
Intraoperative reperfusion assessment of human pancreas allografts using hyperspectral imaging (HSI)
Introduction
While outcomes have continued to improve, pancreas transplantation is still associated with significant potential morbidity and mortality (1). The incidence of anastomosis site leakage after pancreatic transplantation ranges from 5% to 8% and is related to technical problems, such as impaired blood supply and ischemia of the graft (2,3). Adequate oxygen delivery at the tissue level is critical for maintaining graft viability and promoting aerobic metabolism. At present, there are no established techniques to measure tissue oxygenation of pancreas allografts. Routine monitoring of vascular flow and tissue oxygenation is challenging to perform in the clinical setting; however, it may facilitate early detection of events that jeopardize graft perfusion.
Hyperspectral imaging (HSI) has emerged as a novel technology for the measurement of tissue physiology, morphology, and composition (4). HSI provides an enhanced visualization of objects by capturing ultraviolet and infrared wavelength spectra located at either side of visible light of the electromagnetic spectrum. Based on the absorption and reflectance of the analyzed tissue, HSI acquires bidimensional spatial images across the electromagnetic spectrum, which are ultimately formed into a tridimensional data set called the hypercube (5). Computerized imaging procedures subsequently provide pictures of the chemical tissue composition indicating the oxygen saturation (StO2), tissue perfusion (near-infrared perfusion index, NIR), organ hemoglobin index (OHI), and tissue water index (TWI) of the tissue investigated (6).
Several clinical and experimental studies have shown that HSI may have the potential to enhance the surgeon’s visualization beyond gross macroscopic assessment and may have a crucial impact on surgical guidance through tissue characterization (7). A number of studies have recently explored the utility of HSI in assessing tissue perfusion during reconstructive surgery (8), neurosurgery (9), hepatic surgery (10), and gastrointestinal surgery (11).
Holmer et al. (12) have recently described the potential clinical utility of HSI for an experimental study of tissue oxygenation in porcine renal grafts during normothermic machine perfusion. First human data in kidney transplant recipients investigated after organ reperfusion indicate a potential for early prediction of delayed graft function based on parenchymal tissue oxygenation and perfusion parameters (13). However, to date, no studies have investigated the utility of HSI in evaluating the quality of perfusion of pancreatic tissue and transplant anastomosis. Therefore, the objective of the present study was to test intraoperative HSI measurement for real-time monitoring of pancreas grafts, which might help to identify early onset of tissue malperfusion and hypoxia. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-744/rc).
Methods
Patients
For proof of concept, three consecutive simultaneous pancreas kidney allograft recipients were included into this study. All methods were performed in accordance with relevant guidelines and regulations. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Informed consent was obtained from all patients. The study was approved by the institutional review board of the Medical University of Leipzig (AZ 111-16-14032016). Donor and recipient demographics and transplant relevant data were collected.
Surgical technique
The pancreas and kidney grafts were procured according to the guidelines provided by Eurotransplant (ET). As described earlier, the pancreas was explanted in a no-touch technique en bloc with the spleen and duodenum. The spleen and perihepatic fat were removed back-table. An illiac y-graft was used for reconstruction of the superior mesenteric and the splenic artery. The pancreas was transplanted transabdominally using a standard technique with an intraperitoneal location in the right iliac fossa. Arterial anastomosis was performed between the y-graft and the recipient´s common iliac artery using 6-0 Prolene running sutures. The portal vein was anastomosed to the inferior vena cava of the recipient. Exocrine drainage was carried out with a hand-sutured side-to-side duodenojejunostomy 40 cm beyond the flexure of Treitz, as described earlier by our group (14,15).
Kidneys were positioned transabdominally into the left iliacal fossa. Anastomoses of the renal artery and vein were performed to the corresponding external iliac vessels and the ureter was implanted into the bladder according to the Lich-Gregoir technique using a double-J intra-ureteral splint.
Optical remission spectroscopy
Principles of optical remission spectroscopy consist of a physical measurement, in which tissue is irradiated by white light. The incoming light is scattered by different inhomogeneities of the tissue structure and absorbed by different tissue components like hemoglobin and water. This process is influenced by the perfusion state of the organ. Depending on the wavelength, scattering and absorption leads to a different remission spectrum, which is detected by an optical lens mounted on a HSI camera device (Figure 1). The HSI camera system subsequently generates a hyperspectral data-cube, whose information can subsequently be processed with camera specific software.
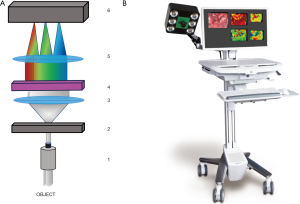
Intraoperative reperfusion assessment of pancreas allografts using HSI
Intraoperative images were acquired using the TIVITA® Tissue Hyperspectral imaging system (Diaspective Vision, Am Salzhaff, Germany). A supplemental video demonstrates how the device is used intraoperatively to take pictures of a reperfused pancreas allograft. For undisturbed image acquisition and data generation the ambient light in the operating room had to be dimmed. The camera system incorporates a high number of spectrally differentiated channels which acquires pictures with a high spectral resolution (5 nm) in the visible and near-infrared range (500–1,000 nm). This scanner is mounted on a moving arm which is brought to the patient from the right side of the operating table. Using a 25-mm focal lens, a constant distance of 30 cm between the object and camera has to be retained during image acquisition. The HSI camera subsequently takes and RGB picture and in parallel computes a pseudo-color image, that represents physiologic parameters like tissue oxygen saturation (StO2%), perfusion (NIR Perfusion index), organ hemoglobin (OHI) and TWI of the recorded tissue area. The maximum relative penetration depth of this HSI system is 6 mm. Quantitative StO2% measurements can either be performed at a depth of up to 1 mm for superficial microcirculation evaluation as well as at a depth of 4–6 mm which corresponds to wavelengths recorded within the near-infrared (NIR) spectrum. HSI can be applied almost “real time” and the acquisition for the hyperspectral image takes less than 10 seconds. All images are stored and further analysis of the hyperspectral data can be performed on the system with the TIVITA® Suite software. In a retrospective analysis, markers representing the region of interest (ROI) were inserted into the pseudo-colored images and the index average from the values inside the ROI were calculated.
Statistical analysis
Statistical analyses were performed using Microsoft excel 2019 and Prism Graph Pad 8 software (Version 8.4.1; GraphPad Software Inc., La Jolla, CA, USA). Parameters are presented as mean/median ± SD/SEM and quartiles. An unpaired two tailed Student’s t-test was used to determine statistical significance. P values <0.05 were considered significant.
Results
Pancreas transplants
Donor and recipient demographics and transplant relevant data are provided in Tables 1,2. In short, all patients had insulin dependent diabetes mellitus type 1 (IDDM1) with end stage renal disease (ESRD) requiring hemodialysis. All patients were listed by ET for simultaneous pancreas kidney transplantation. Reported organ quality was good for all, pancreas and kidney allografts. Immunosuppressive therapy comprised an induction therapy with the interleukin-2 receptor antagonist basiliximab followed by a triple maintenance immunosuppression of tacrolimus, mycophenolate-mofetil and tapered steroids. One patient developed a Banff 1a rejection which was responsive to steroid treatment. Otherwise the postoperative course was uneventful. No anastomotic leaks were recorded.
Table 1
| Variable | Donor | ||
|---|---|---|---|
| A | B | C | |
| Age, years | 35 | 22 | 18 |
| Gender | Female | Male | Male |
| Size, cm | 175 | 170 | 176 |
| Weight, kg | 75 | 60 | 70 |
| BMI, kg/m2 | 24.5 | 20.8 | 22.6 |
| Cause of death | Cerebrovascular event | Intracranial injury | Cerebral oedema |
| Laboratory tests | |||
| Sodium, mmol/L | 147 | 143 | 160 |
| Potassium, mmol/L | 4.0 | 3.9 | 3.6 |
| Serum creatinine, µmol/L | 44.0 | 88.4 | 62 |
| Lipase, U/L | 23 | 15 | 31 |
| Anti-CMV-IgG | Positive | Negative | Negative |
| Secondary disease | Hypothyroidism | None | None |
BMI, body mass index; CMV, cytomegalovirus.
Table 2
| SPKT | 1 | 2 | 3 |
|---|---|---|---|
| Recipient | |||
| Age, years | 39 | 42 | 48 |
| Gender | Female | Female | Female |
| Size, cm | 163 | 165 | 160 |
| Weight, kg | 58 | 62 | 59 |
| BMI, kg/m2 | 21.8 | 22.8 | 23.0 |
| Primary disease | IDDM I, diabetic nephropathy | IDDM I, diabetic nephropathy | IDDM I, diabetic nephropathy |
| Dialysis type | Hemodialysis | Hemodialysis | Hemodialysis |
| Dialysis duration, months | 44 | 69 | 53 |
| Time on the waiting list, months | 51 | 63 | 35 |
| Anti-CMV-IgG | Positive | Positive | Positive |
| Graft/transplant | |||
| Duration of surgery, minutes | 314 | 358 | 312 |
| Pancreas | |||
| Organ quality | Good | Good | Good |
| Implantation side | Right | Right | Right |
| Exocrine drainage | Duodenojejunostomy | Duodenojejunostomy | Duodenojejunostomy |
| CIT, minutes | 462 | 797 | 313 |
| Anastomosis, min | 30 | 28 | 30,0 |
| Kidney | |||
| Organ quality | Good | Good | Good |
| Donated side | Left | Left | Left |
| Implantation side | Left | Left | Left |
| Artery | |||
| Number | 1 | 2 | 2 |
| Patch | Yes | Yes | Yes |
| Vein | |||
| Number | 1 | 1 | 1 |
| Patch | Yes | Yes | Yes |
| Ureteral anastomosis | Ureterocystostomy | Ureterocystostomy | Ureterocystostomy |
| CIT, minutes | 583 | 904 | 426 |
| Anastomosis, min | 30 | 38 | 47 |
| Immunosuppression | |||
| Induction therapy | Basiliximab | Basiliximab | Basiliximab |
| CNI | Tacrolimus | Tacrolimus | Tacrolimus |
| AP drug | MMF | MMF | MMF |
| Postoperative | |||
| ICU, days | 6 | 5 | 4 |
| Complications | Secondary wound healing, lymphocele | None | None |
| Hospital stay, days | 48 | 15 | 16 |
| Rejection | |||
| Grade | Banff-1a, kidney | None | None |
1, recipient 1; 2, recipient 2; 3, recipient 3. BMI, body mass index; CMV, cytomegalovirus; IDDM 1, Insulin dependent diabetes mellitus type 1; ICU, intensive care unit; SPKT, simultaneous pancreas-kidney transplantation.
Hyperspectral assessment of pancreas parenchyma after transplantation
Hyperspectral images of pancreatic allografts were acquired 15 minutes after reperfusion (Figure 2). There were no remarkably differences of pancreas tissue parameters between the three patients/cases. Pancreas grafts showed a high and homogeneous oxygen saturation (StO2: 92.6%±10.45%) On average, mean/median NIR was 0.54±0.07 [I (Index)], OHI 0.84±0.12 [I] and TWI 0.63±0.11 [I], respectively. Perfusion deficits within the organ were not detected. Furthermore, no differences in head corpus and tale perfusion were detected (data not shown).
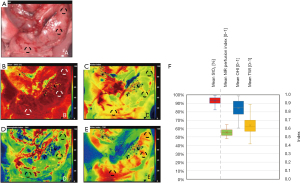
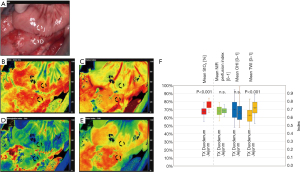
HSI of intestinal anastomoses after transplantation
Intraoperative HSI of the intestinal anastomosis displayed significant differences of oxygen saturation (StO2 duodenum 67.46%±5.60% vs. jejunum: 75.93%±4.71% P<0.001), and TWI {TWI duodenum: 0.63±0.09 [I] vs. TWI jejunum: 0.72±0.09 [I], P<0.001} between donor duodenum and recipients’ jejunum. The other two-color coded images (NIR and OHI) did not display remarkably differences {NIR duodenum: 0.68±0.06 [I] vs. NIR jejunum: 0.69±0.04 [I], P=0.747; OHI duodenum: 0.70±0.12 [I] vs. OHI jejunum: 0.68±0.13 [I], P=0.449}. Representative images and distribution of the four calculated tissue parameters are illustrated in Figure 3.
Discussion
In this study, we describe for the first time the use of HSI in patients receiving a simultaneous kidney-pancreas transplantation to evaluate the perfusion quality of the pancreatic tissue and the intestinal graft anastomosis. Using this novel technology, our main purpose was to augment the surgeon’s visual armamentarium for the assessment of the pancreas allograft and the duodenojejunal anastomosis after reperfusion, before wound closure is performed. In addition, we aimed to quantify the oxygenation and perfusion status of the different components of the pancreas graft as well as the intestinal anastomosis and lay the foundation for further larger cohort studies.
During solid organ transplantation, adequate delivery of oxygen at the tissue level is critical for maintaining graft viability and promoting aerobic metabolism (16-19). Real-time monitoring of vascular flow and tissue oxygenation indicates viable tissue and facilitates early detection of malperfusion. Currently, available technology is inconvenient for routine clinical use. The simplicity of HSI makes it attractive for clinical use, but the lack of a “gold standard” technique for measuring tissue oxygen saturation makes validation challenging.
Previous studies have applied polarographic electrodes for monitoring of pancreatic tissue oxygenation in patients undergoing pancreaticoduodenectomy (20). However, these invasive sensors do only provide localized tissue information and increase the risk of tissue damage. In contrast, HSI is non-invasive and provides the surgeon with no-touch advanced visualization, extended into the infrared and near-infrared wavelength regions, creating a specific “oxygenation map” of the whole organ. HSI oxygenation mapping allows for assessment of critical and/or jeopardized areas after reperfusion and provides information about the heterogeneity of oxygen supply in the tissue. Our preclinical studies in porcine kidney grafts subject to machine perfusion showed that the oxygenation map provided by HSI detected arterial occlusions and differentiated between perfused and non-perfused tissue regions (12). In our recent study on human kidney allografts, we were able to proof that intraoperative HSI of the kidney parenchyma early after reperfusion allowed for identification of grafts which subsequently developed a delayed graft function. Our conclusions were built on characteristic differences in tissue oxygenation and near infrared perfusion indices, measured in the parenchyma of the transplanted organs. Our technique furthermore allowed for the assessment of ureter tissue viability which facilitated a better estimation at which distance from the renal pelvis a save uretreo-cystostomy of a sufficiently perfused ureter segment could be performed (13).
In recent years indocyanine green fluorescence staining has gained popularity in various surgical disciplines including liver surgery (21-23), biliopancreatic surgery (24), upper Gi surgery (25,26), colorectal surgery (27), thoracic surgery (28) and transplant surgery (29). As a matter of fact, the use of ICG fluorescence angiography, and near-infrared spectroscopy showed promising results in duodenum-preserving pancreatic head resection providing intraoperative demarcation of pancreatic tumors (30) as well as anatomic liver resections performed with vascular inflow control (31). A first case report of duodenal perfusion assessment in pancreas transplantation by means of ICG fluorescence was published in 2014 (32). A recent study described the application of ICG fluorescence angiography during liver and pancreas transplantation in a larger series of patients (33). The detection of intraoperative perfusion defects allowed for real time modification of technical strategies. In fact, the detection of an ischemic area of a duodenal stump in a pancreas allograft could promptly be addressed and a consecutive synchronous second resection of the ischemic area led to an uneventful healing of the duodenal-enteric anastomosis. ICG is a fluorescent dye, which following intravenous injection binds to albumin and gets removed from the blood stream by the liver. In this context it has been traditionally used for liver function assessments prior major liver resections, since the removal rate of ICG provided a quantitative estimate of liver mass (34). Although very rare, severe adverse anaphylactic reactions to intravenous ICG application have been reported in the literature (35).
When compared to ICG angiography, the near infrared perfusion index gained by HSI is capable to provide all the visual and quantitative information on organ perfusion. On top, our novel technology is capable to provide a visual measurement of tissue oxygenation, tissue hemoglobin concentration and tissue water concentration. In this regard it does not require the introduction of a fluorescent agent and hence qualifies to be designated as a true non-invasive, no-touch examination method.
Our group recently demonstrated that HSI could help surgeons to visualize intestinal perfusion and help determine resection margins during colorectal resection (36). In this study, we did not experience any future anastomotic leakage, which is in line with the hyperspectral data of our pancreas transplant duodenojejunostomies which displayed comparable tissue perfusion indices and homogeneous oxygen saturation map. In case of abnormal hyperspectral tissue oxygenation and perfusion the transplant surgeon has the real time option to either control arterial inflow and venous outflow improve organ positioning and in case revise the anastomosis or worst case remove the graft to avoid devastating complications. The application of an exocrine bladder drainage might furthermore be an alternative to the entero-enteric drainage. However, if this method is superior in case of ischemia of the transplanted duodenum remains speculative. In case of exclusion of any surgical errors with regard to vascular inflow and outflow an adjustment of postoperative anticoagulation therapy might be considered as well.
Main limitations of HSI comprise the fact that this technique can only be applied intraoperatively and no hyperspectral organ assessment can be performed after wound closure. As a matter of fact, a second glance to the transplanted organ would require redo-surgery. Furthermore, in accordance with the current available technology, HSI is only feasible in open surgical procedures, however a laparoscopic device should be available soon.
At this point we can conclude that HSI offers a real-time examination of the pancreatic graft and anastomotic site. Intraoperative measurement of the perfusion of the parenchyma and intestinal anastomosis during pancreas transplantation might help evaluating regional tissue injury and microcirculation following pancreas reperfusion. Our results suggest that HSI may be a useful tool for optimizing pancreatic transplantation by guiding the surgeon with the help of an objective decision aid that may improve surgical accuracy and lower complications. Our present study confirmed feasibility of the intro-operative imaging of the pancreas/duodenal graft with optimal perfusion. The value of the detection of hypo perfused areas remains to be assessed in future applications.
Acknowledgments
We acknowledge support from the German Research Foundation (DFG), and Leipzig University within the program of Open Access Publishing.
Funding: Part of the technical equipment for data analysis was funded by Project nr: BGAAF-0839.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-744/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-744/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-20-744/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics committee of the University of Leipzig (No. AZ: Nr: 111–16 14,032,016) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dean PG, Kukla A, Stegall MD, et al. Pancreas transplantation. BMJ 2017;357:j1321. [Crossref] [PubMed]
- Pieroni E, Napoli N, Lombardo C, et al. Duodenal graft complications requiring duodenectomy after pancreas and pancreas-kidney transplantation. Am J Transplant 2018;18:1388-96. [Crossref] [PubMed]
- Messner F, Bosmuller C, Oberhuber R, et al. Late recurrent bleeding episodes from duodenojejunostomy after pancreas transplantation. Clin Transplant 2018;32:e13350. [Crossref] [PubMed]
- Lu G, Fei B. Medical hyperspectral imaging: a review. J Biomed Opt 2014;19:10901. [Crossref] [PubMed]
- Kulcke A. Device and method for recording a hyperspectral image. German Patent 2015;EP2851662A2. Available online: https://patents.google.com/patent/EP2851662A3/de
- Kulcke A, Holmer A, Wahl P, et al. A compact hyperspectral camera for measurement of perfusion parameters in medicine. Biomed Tech (Berl) 2018;63:519-27. [Crossref] [PubMed]
- Shapey J, Xie Y, Nabavi E, et al. Intraoperative multispectral and hyperspectral label-free imaging: A systematic review of in vivo clinical studies. J Biophotonics 2019;12:e201800455. [Crossref] [PubMed]
- Jones GE, King VA, Yoo A, et al. Use of New Technologies in Implant-Based Breast Reconstruction. Semin Plast Surg 2019;33:258-63. [Crossref] [PubMed]
- Bravo JJ, Olson JD, Davis SC, et al. Hyperspectral data processing improves PpIX contrast during fluorescence guided surgery of human brain tumors. Sci Rep 2017;7:9455. [Crossref] [PubMed]
- Sucher R, Athanasios A, Kohler H, et al. Hyperspectral Imaging (HSI) in anatomic left liver resection. Int J Surg Case Rep 2019;62:108-11. [Crossref] [PubMed]
- Barberio M, Longo F, Fiorillo C, et al. HYPerspectral Enhanced Reality (HYPER): a physiology-based surgical guidance tool. Surg Endosc 2020;34:1736-44. [Crossref] [PubMed]
- Holmer A, Tetschke F, Marotz J, et al. Oxygenation and perfusion monitoring with a hyperspectral camera system for chemical based tissue analysis of skin and organs. Physiol Meas 2016;37:2064-78. [Crossref] [PubMed]
- Sucher R, Wagner T, Kohler H, et al. Hyperspectral Imaging (HSI) of Human Kidney Allografts. Ann Surg 2020; Epub ahead of print. [Crossref] [PubMed]
- Sucher R, Rademacher S, Jahn N, et al. Effects of simultaneous pancreas-kidney transplantation and kidney transplantation alone on the outcome of peripheral vascular diseases. BMC Nephrol 2019;20:453. [Crossref] [PubMed]
- Hau HM, Jahn N, Brunotte M, et al. Short and long-term metabolic outcomes in patients with type 1 and type 2 diabetes receiving a simultaneous pancreas kidney allograft. BMC Endocr Disord 2020;20:30. [Crossref] [PubMed]
- Maglione M, Oberhuber R, Cardini B, et al. Donor pretreatment with tetrahydrobiopterin saves pancreatic isografts from ischemia reperfusion injury in a mouse model. Am J Transplant 2010;10:2231-40. [Crossref] [PubMed]
- Sucher R, Gehwolf P, Kaier T, et al. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/reperfusion-associated damage. Transpl Int 2009;22:922-30. [Crossref] [PubMed]
- Sucher R, Hautz T, Mohr E, et al. Sodium Sulfite Exacerbates Allograft Vasculopathy and Affects Tryptophan Breakdown in Murine Heterotopic Aortic Transplantation. Oxid Med Cell Longev 2019;2019:8461048. [Crossref] [PubMed]
- Sucher R, Gehwolf P, Oberhuber R, et al. Tetrahydrobiopterin protects the kidney from ischemia-reperfusion injury. Kidney Int 2010;77:681-9. [Crossref] [PubMed]
- Koong AC, Mehta VK, Le QT, et al. Pancreatic tumors show high levels of hypoxia. Int J Radiat Oncol Biol Phys 2000;48:919-22. [Crossref] [PubMed]
- Lwin TM, Hoffman RM, Bouvet M. Fluorescence-guided hepatobiliary surgery with long and short wavelength fluorophores. Hepatobiliary Surg Nutr 2020;9:615-39. [Crossref] [PubMed]
- Sucher R, Brunotte M, Seehofer D. Indocyanine green fluorescence staining in liver surgery. Chirurg 2020;91:466-73. [Crossref] [PubMed]
- Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary Surg Nutr 2016;5:322-8. [Crossref] [PubMed]
- Baiocchi GL, Diana M, Boni L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J Gastroenterol 2018;24:2921-30. [Crossref] [PubMed]
- Nakashima Y, Saeki H, Yukaya T, et al. Blood Flow Assessment with Indocyanine Green Fluorescence Angiography for Pedicled Omental Flap on Cervical Esophagogastric Anastomosis after Esophagectomy. J Am Coll Surg 2016;222:e67-9. [Crossref] [PubMed]
- Tajima Y, Yamazaki K, Masuda Y, et al. Sentinel node mapping guided by indocyanine green fluorescence imaging in gastric cancer. Ann Surg 2009;249:58-62. [Crossref] [PubMed]
- Jansen-Winkeln B, Germann I, Kohler H, et al. Comparison of hyperspectral imaging and fluorescence angiography for the determination of the transection margin in colorectal resections-a comparative study. Int J Colorectal Dis 2021;36:283-91. [Crossref] [PubMed]
- Okusanya OT, Hess NR, Luketich JD, et al. Infrared intraoperative fluorescence imaging using indocyanine green in thoracic surgery. Eur J Cardiothorac Surg 2018;53:512-8. [Crossref] [PubMed]
- Figueroa R, Golse N, Alvarez FA, et al. Indocyanine green fluorescence imaging to evaluate graft perfusion during liver transplantation. HPB (Oxford) 2019;21:387-92. [Crossref] [PubMed]
- Newton AD, Predina JD, Shin MH, et al. Intraoperative Near-infrared Imaging Can Identify Neoplasms and Aid in Real-time Margin Assessment During Pancreatic Resection. Ann Surg 2019;270:12-20. [Crossref] [PubMed]
- Sucher R, Rademacher S, Lederer A, et al. Laparoscopic Left Hemihepatectoy Applying Intraoperative Indocyanine Green Fluorescence Detection Counter Perfusion Method for Visualization. Zentralbl Chir 2020;145:135-7. [PubMed]
- Garcia-Roca R, Walczak D, Tzvetanov I, et al. The application of indocyanine green to evaluate duodenal perfusion in pancreas transplantation. Am J Transplant 2014;14:226-8. [Crossref] [PubMed]
- Panaro F, Benedetti E, Pineton de Chambrun G, et al. Indocyanine green fluorescence angiography during liver and pancreas transplantation: a tool to integrate perfusion statement's evaluation. Hepatobiliary Surg Nutr 2018;7:161-6. [Crossref] [PubMed]
- Moody FG, Rikkers LF, Aldrete JS. Estimation of the functional reserve of human liver. Ann Surg 1974;180:592-8. [Crossref] [PubMed]
- Garski TR, Staller BJ, Hepner G, et al. Adverse reactions after administration of indocyanine green. JAMA 1978;240:635. [Crossref] [PubMed]
- Jansen-Winkeln B, Holfert N, Kohler H, et al. Determination of the transection margin during colorectal resection with hyperspectral imaging (HSI). Int J Colorectal Dis 2019;34:731-9. [Crossref] [PubMed]


