
Prospective evaluation of 18F-FDG positron emission tomography in the preoperative staging of patients with hepatic colorectal metastases
Introduction
Hepatectomy has become accepted as the standard, potentially curative therapy for patients with metastatic colorectal cancer. Contemporary series have demonstrated 5-year overall survival estimates of 37% to 58% after partial hepatectomy (1-5). This success is clearly referable to significant improvements in preoperative selection, operative and perioperative management, and adjuvant chemotherapy. With strategies like portal vein embolization, segment-oriented resection, and tumor ablation, surgical therapy can now be offered to patients with ever-increasing degrees of tumor burden (6). However, up to a third of patients are still found to have technically unresectable disease at the time of operative exploration (7-9). Clearly, further refinements in our preoperative selection of patients undergoing operative intervention are needed.
[18F]2-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) has emerged as a diagnostic tool widely used in the staging and follow-up of patients with cancer (10-14). FDG-PET performed after systemic administration of the glucose analog FDG takes advantage of the enhanced glycolytic activity exhibited by neoplasms. FDG taken up by neoplastic cells via GLUT transporters is phosphorylated by hexokinase into the metabolite [18F]-FDG-6-phosphate, which cannot be further metabolized and accumulates intratumorally. Detection by PET of 511-keV photons released by [18F]-FDG trapped by the high glucose transport and high expression of hexokinase therefore permits selective visualization of hypermetabolic tumor tissue. Retrospective and prospective investigations have suggested that use of FDG-PET in preoperative evaluation may refine our ability to properly select patients for operative intervention (12-25). However, these investigations to date have been limited by few studies designed to assess the relative role of FDG-PET compared to other standard cross-sectional imaging. There have also been few studies that examined the accuracy and utility of FDG-PET for disease outside or within the liver, where background uptake of FDG is high. The current study is a prospective study with blinded readings of FDG-PET and cross-sectional imaging that aims to refine the roles of this test in management of the surgical patient with hepatic colorectal metastases.
Methods
Patients and interventions
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Memorial Sloan-Kettering Cancer Center and informed consent was taken from all individual participants. Institutional review board approval was obtained for a prospective assessment of the clinical utility of preoperative FDG-PET imaging for patients with hepatic colorectal metastases. Patients with synchronous or metachronous hepatic colorectal metastases undergoing evaluation at Memorial Sloan-Kettering Cancer Center for potential hepatic metastasectomy were eligible for participation in this trial. Age less than 18 years, inability to provide informed consent, and pregnancy were exclusion criteria for entry. After undergoing a complete history and physical examination, participants underwent imaging with conventional helical abdominopelvic computed tomography (CT) with oral and intravenous contrast administration, CT arterial portography (CTAP), and FDG-PET. Both conventional contrast-enhanced helical CT and CTAP were employed in order to optimize the diagnostic efficacy of CT in the detection of intrahepatic and extrahepatic disease (26). All scans were performed within 3 weeks of surgery. Additional diagnostic interventions were performed at the discretion of the attending surgeon. Each case was then reviewed with all relevant imaging and other diagnostic studies by the multidisciplinary Hepatobiliary Disease Management Team. Patients found to have clear evidence of radiographically unresectable disease did not undergo surgical therapy. All other patients underwent operative intervention, which began with careful exploration of the abdominopelvic contents. Any areas of abnormality detected on preoperative FDG-PET scans but not seen by other imaging were intentionally explored, and all abnormal tissues were biopsied.
Interpretation of CT, CTAP or FDG-PET was performed by radiologists blinded to the other modality, and results were recorded prospectively. Five experienced radiologists prospectively assessed how well FDG-PET, CT, or CTAP revealed metastatic involvement by cancer: FDG-PET (TA), CT (LHS), CTAP (AC, KTB). In all cases, the radiologists knew that the patient had colorectal carcinoma and suspected liver metastases but were unaware of the results of any other diagnostic procedure (e.g., magnetic resonance imaging, helical CT, or FDG-PET). The number, size, and location (according to the Couinaud numbering system) of each focal lesion were noted. Each lesion was assigned a score from 0 to 4, with 0 being “definitely not cancer” and 4 being “definitely cancer”.
All surgical liver procedures were performed by one of five experienced hepatic surgeons. Partial hepatectomy was performed using standard operative techniques previously described (27), and all operative and pathologic findings were prospectively recorded. During operation, the liver was carefully examined both by palpation and by intraoperative sonography to confirm the number and size of the metastases, define their relationship with vascular landmarks, and look for occult liver metastases. The hepatic surgeon was aware of the results of the FDG-PET. Intraoperative sonography was performed with a flexible system, SSD-1100 (Aloka, Tokyo, Japan), using a 5- and 7.5-MHz intraoperative probe.
In the postoperative period, preoperative imaging and diagnostics, operative findings and procedures, and pathologic results were reviewed and summarized by the attending surgeon, who recorded whether CT or FDG-PET results were concordant with operative and pathologic findings, and whether FDG-PET independently changed the management of the patient.
This is an NIH-funding prospective trial (NIH-R01CA/DK80982-01) to assess the usefulness of FDG-PET scanning in this clinical setting and this paper is the final report with full ten-year follow-up for assessment of false negatives and for survival.
FDG-PET
All patients were imaged on a state-of-the-art, high resolution, high sensitivity dedicated BGO PET system, the GE Advance (GE Medical Systems, Milwaukee, WI, USA), after injection of 10–15 mCi (370–555 MBq) 18F-FDG. Iteratively reconstructed images of the FDG-PET scans were read with the nuclear medicine physician blinded to the results of other scanning. FDG-PET results were quantified by calculating the maximum standardized uptake value (SUV) for lesions detected (28). All modalities were also graded on a 5-point ordinal confidence scale (0–4), with a score of 0–2 classified as a negative scan and a score of 3–4 regarded as a positive scan.
At the time of surgery, the number and site of each tumor within the liver were recorded. Serial thin slices of the resected specimen were then examined and all tumor nodules identified (and confirmed as cancers by histopathology). These pathologic findings were correlated with the blinded FDG-PET reading.
Helical CT
Helical CT was performed using the GE Lightspeed (GE Medical Systems) system after oral administration of 600–800 mL contrast and intravenous administration of Omnipaque. CTAP was performed after placing a selective catheter into the proximal superior mesenteric artery (SMA). A total of 180–190 mL non-ionic contrast was administered through the catheter, after which helical imaging was obtained through the liver.
CTAP
CTAP was performed immediately following routine visceral angiography, with the catheter left deep enough in the SMA to preclude dislodgement during patient transfer. In the case of accessory or replaced hepatic vessels arising from the SMA, care was taken to place the catheter sufficiently distal to the aberrant hepatic artery to avoid reflux during CTAP. For the portogram, iohexol 140 was injected at 3 mL/s for a total volume of 180–190 mL (the injector holds a maximum of 200 mL; some is lost during filling and hooking up the catheter). Imaging was initiated 40–60 s after the injection start, beginning at the top of the liver and scanning caudally. Seven-millimeter-thick contiguous axial images were acquired in a helical fashion (pitch =1.0). After an additional 20–30 s delay, 7-mm-thick images were acquired in either the same or the reverse direction, still in helical fashion (pitch =1.0) (Obs.: the protocol for direction of scanning changed during the study, initially the second series was acquired from caudad to cephalad; part way through the study this switched so that all series were performed from caudad to cephalad). The patient returned 3.5–4 hours later for follow-up scanning without additional contrast; 7-mm-thick images were obtained helically (pitch still =1.0) in a craniocaudad direction. Each set of helical images was obtained during a single breath hold.
Pathology
Pathologic specimens were sliced at 5-mm intervals, and direct radiologic-pathologic correlation was obtained. Each detected lesion was measured and examined microscopically. The results of this radiologic-pathologic correlation and those of the surgical palpation and intraoperative sonographic examination of nonresected portions of the liver constituted the gold standard of reference of our study.
Un-resectable disease identified by pre-operative scanning
When lesions were identified by pre-operative scanning that denoted unresectable disease, lesions were confirmed by biopsy or follow-up scanning to show progression. Full 10-year follow-up of patients was performed to allow long-term scanning to identify false negatives.
Statistics
Each lesion detected at pathology and reported on the data sheet as metastasis in the same location with similar size was considered true-positive. Each lesion detected at pathology and missed at the imaging studies was considered false-negative. Lesions considered metastasis by imaging and subsequently not shown to represent tumor at surgical or histologic examination were considered false-positive. Sensitivity was defined as the number of metastases correctly detected with the imaging techniques divided by the number of metastatic lesions identified at pathologic and surgical examination. The false-positive rate was defined as the number of false-positive lesions detected with helical CT or CTAP divided by the total number of lesions (true-positive plus false-positive) detected with each technique. The positive predictive value was defined as the number of metastases correctly detected with imaging divided by the total number of lesions considered metastatic with imaging. Lack of a lesion on imaging or surgical/pathological confirmation was counted as true-negative. Each lobe was considered only once for the purpose of counting false- and true-negatives. The sample size was prospectively chosen to allow confident discrimination of outcomes.
For patients undergoing operative intervention, CT or FDG-PET imaging was considered to be concordant with operative and pathologic findings only if every abnormal lesion interpreted to be metastatic disease on imaging was ultimately confirmed to harbor metastatic colorectal adenocarcinoma, and no other foci of disease were identified at the time of operative exploration and pathologic analysis. Cases of discordance between radiologic and operative/pathologic findings were recorded as false-negatives, false-positives, or both. FDG-PET was retrospectively determined to have changed therapy if diagnostic or therapeutic decisions (e.g., the decision to not operate) or interventions (e.g., biopsy) were rendered based on radiologic findings seen on FDG-PET imaging alone (and not seen on CT imaging).
A preoperative clinical risk score (CRS) previously described and used to stratify the likelihood of oncologic recurrence following partial hepatectomy was calculated for each patient (1). This score is based on the presence of node-positivity in the primary lesion, disease-free interval from resection of the primary tumor to metastasis less than 12 months, presence of multiple hepatic tumors, maximal hepatic tumor size greater than 5 cm, and carcinoembryonic antigen level greater than 200 ng/mL.
For each extra-hepatic site, the sensitivity, specificity, positive predictive value, and negative predictive value of the dichotomized ratings from each imaging modality were calculated using SAS statistical software version 9.2. These statistics were also calculated for hepatic specimens overall, by whether the patient was undergoing chemotherapy or not, and by size categories (≤1 cm, >1 cm, between 1 cm and 5 cm). The standard errors were adjusted for the clustering of specimens within patients using the “cluster” statement in PROC SURVEYFREQ, which uses the Taylor series method to estimate variance from the variance among the clusters. The McNemar test was employed to test whether the sensitivities and specificities, respectively, of any two imaging modalities were significantly different, adjusted for clustering (29). To assess the accuracy of each imaging modality at a more refined level, the radiologist-assigned scores for each image, ranging from 0 to 4, were used to plot receiver operating characteristic (ROC) curves and to calculate the area under the curves (AUC) (30).
Results
Patient demographics and clinical outcomes
One hundred and twenty-five patients were enrolled into this prospective study. The median age was 61 years (range, 30–82 years), and 56 out of the 125 patients were female (45%).
Treatment outcomes are summarized in Figure 1. Of the 125 patients enrolled, 24 (19%) were found to have clear radiographic evidence of unresectability and did not undergo operative intervention. Seventeen of these patients (71%) were deemed unresectable due to extrahepatic disease; the remaining seven (29%) were unresectable due to extent of intrahepatic disease. Of the remaining 101 (81%) who underwent operative intervention, 26 were intraoperatively found to be unresectable and did not undergo metastasectomy. Eleven cases were due to extrahepatic disease, 14 were due to intrahepatic extent of disease, and one case was due to refractory intraoperative hypotension. Among the entire cohort of 125 patients, the overall resectability rate of 60%; among the 101 patients undergoing operative exploration, the intraoperative resectability rate was 74%. The overall sensitivity of preoperative radiographic determination of irresectability was 48%.
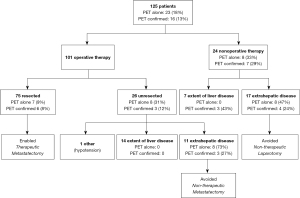
Findings seen on FDG-PET alone altered therapy for 23 of the 125 patients (18%) (Figure 1). PET confirmed other radiologic findings in 16 cases (13%), for an overall influence on therapy in 39 cases (31%). Of the 28 cases of extrahepatic disease that ruled out metastatectomy, 23 cases were FDG-avid (Figure 1).
For the 24 patients determined to be radiographically irresectable and therefore treated nonoperatively, FDG-PET identified the reason(s) for unresectability in 15 (63%), and findings seen on FDG-PET alone were the sole determinant of inoperability in eight (33%). Seventeen of the nonoperatively managed patients were unresectable due to extrahepatic disease; FDG-PET identified these foci of extrahepatic disease in 12 (71%), and FDG-PET findings alone identified extrahepatic disease in eight (47%). Seven of the nonoperatively managed patients were determined to be unresectable due to extent of intrahepatic disease; FDG-PET identified the reason for unresectability in three (43%), but none of these cases were recognized based on FDG-PET findings alone.
Among the 101 patients who underwent operative intervention, FDG-PET findings alone changed therapy in 15 cases (15%). The impact of FDG-PET on clinical management was more pronounced in the subset of 26 patients who were intraoperatively found to be unresectable; in eight of these cases (31%), initial exploration directed toward areas of abnormality detected only on preoperative FDG-PET imaging confirmed unresectability, and partial hepatectomy was avoided. In all eight cases, unresectability was due to extrahepatic disease. In another three cases, FDG-PET along with other imaging identified evidence of unresectable extrahepatic disease in five cases.
Among the 75 patients who underwent metastasectomy, findings seen on FDG-PET alone changed therapy in seven cases (9%) by demonstrating that extrahepatic or intrahepatic lesions suspected to be cancer on CT (that would have resulted in unresectability) were hypometabolic and unlikely to be foci of metastatic disease. In another six (8%) cases, FDG-PET confirmed that CT-stable lesions were not cancer.
When all patients with unresectable disease (determined both preoperatively and intraoperatively) were pooled, 28 of 50 cases (56%) were due to extrahepatic disease, and FDG-PET findings solely changed therapies in 16 cases (57%) and influenced therapy in seven others (25%). Twenty-one of the 50 unresectable cases (42%) were due to extent of intrahepatic disease; FDG-PET did not solely change therapy in any of these cases. Of the 23 patients for whom FDG-PET changed therapy, eight were spared unnecessary operative intervention due to extrahepatic metastases not seen on CT imaging, eight were spared unnecessary partial hepatectomy due to intraoperatively confirmed extrahepatic metastases that had not been suspected based on CT imaging, and seven were able to undergo partial hepatectomy because FDG-PET findings confirmed that lesions suspected by CT to be unresectable cancer were not foci of metastatic disease.
Extrahepatic disease
Clearly extrahepatic disease was where FDG-PET had the most yield. There were 45 lung lesions, 66 sites of lymph nodes, 44 peritoneal lesions, and 21 sites of extrahepatic organs (including colon, rectum, spleen) seen on the various scanning modalities. Of these, 11 lung lesions, 18 lymph nodes, and 23 peritoneal sites were confirmed cases of malignancy in extrahepatic organs. The prevalence of detection at each site as well as the sensitivity, specificity, positive predictive value, and negative predictive values are presented in Table 1. FDG-PET was 80–90% sensitive for extrahepatic cancer, and 70–90% specific. Of note, FDG-PET was clearly the most sensitive modality for extrahepatic cancer at all sites, and CTAP was the least sensitive test. CTAP was highly specific, however, reflecting the conservative nature with which this scanning modality is read outside the liver.
Table 1
| Test | Extrahepatic site | n | Test prevalence | (SE*) | Pathology prevalence | (SE) | Sensitivity | (SE) | Specificity | (SE) | PPV | (SE) | NPV | (SE) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | Lung | 45 | 86.67 | 5.14 | 24.44 | 6.53 | 81.82 | 11.76 | 11.76 | 5.61 | 23.07 | 6.88 | 66.67 | 19.47 |
| Lymph node | 66 | 63.64 | 5.97 | 27.27 | 5.52 | 38.89 | 11.58 | 27.08 | 6.46 | 16.66 | 5.79 | 54.17 | 10.25 | |
| Peritoneum | 44 | 54.55 | 7.59 | 52.27 | 7.62 | 47.83 | 10.54 | 38.10 | 10.72 | 45.83 | 10.29 | 40.00 | 11.08 | |
| Organ | 21 | 61.90 | 10.86 | 28.57 | 10.10 | 50.00 | 20.92 | 33.33 | 12.47 | 23.08 | 11.97 | 62.50 | 17.54 | |
| CTAP | Lung | 45 | 15.56 | 5.34 | 24.44 | 6.53 | 27.27 | 13.58 | 88.24 | 5.42 | 42.85 | 18.92 | 78.95 | 6.69 |
| Lymph node | 66 | 19.70 | 4.93 | 27.27 | 5.52 | 16.67 | 8.85 | 79.17 | 5.91 | 23.07 | 11.78 | 71.70 | 6.24 | |
| Peritoneum | 44 | 4.55 | 3.18 | 52.27 | 7.62 | 4.35 | 4.30 | 95.24 | 4.70 | 50.00 | 35.76 | 47.62 | 7.80 | |
| Organ | 21 | 14.29 | 7.82 | 28.57 | 10.10 | 16.67 | 15.59 | 86.67 | 8.99 | 33.33 | 27.89 | 72.22 | 10.82 | |
| FDG-PET | Lung | 45 | 28.89 | 6.90 | 24.44 | 6.53 | 90.91 | 8.77 | 91.18 | 4.93 | 76.92 | 11.82 | 96.88 | 3.11 |
| Lymph node | 66 | 39.39 | 6.06 | 27.27 | 5.52 | 83.33 | 8.85 | 77.08 | 6.11 | 57.69 | 9.76 | 92.50 | 4.20 | |
| Peritoneum | 44 | 63.64 | 7.34 | 52.27 | 7.62 | 78.26 | 8.70 | 52.38 | 11.02 | 64.28 | 9.16 | 68.75 | 11.72 | |
| Organ | 21 | 42.86 | 11.07 | 28.57 | 10.10 | 83.33 | 15.59 | 73.33 | 11.70 | 55.55 | 16.97 | 91.67 | 8.18 |
*, all standard errors are corrected for the clustering of lesions within patients using the “cluster” statement in SAS PROC SURVEYFREQ, with the exception of the Kappa SE, which is uncorrected. CT, computed tomography; CTAP, CT arterial portography; FDG-PET, [18F]2-fluoro-2-deoxyglucose positron emission tomography; PPV, positive predictive value; NPV, negative predictive value.
Comparison of accuracy of various tests is shown in Table 2. FDG-PET was never significantly outperformed by CT, and only in one case (specificity in the peritoneum) was significantly worse than CTAP. In contrast, FDG-PET had significantly higher sensitivity than both CT and CTAP in the lymph nodes and peritoneum. FDG-PET also had significantly better specificity than CT in the lung and lymph nodes.
Table 2
| Extrahepatic site | Scan | Sensitivity (%) | Chi-square (df =1) | P value | Specificity (%) | Chi-square (df =1) | P value |
|---|---|---|---|---|---|---|---|
| Lung | CT | 81.82 | 6.00 | 0.014 | 11.76 | 24.14 | <0.0001 |
| CTAP | 27.27 | 88.24 | |||||
| CT | 81.82 | 0.33 | 0.564 | 11.76 | 20.83 | <0.0001 | |
| FDG-PET | 90.91 | 91.18 | |||||
| CTAP | 27.27 | 7.00 | 0.008 | 88.24 | 0.14 | 0.706 | |
| FDG-PET | 90.91 | 91.18 | |||||
| Lymph node | CT | 38.89 | 2.00 | 0.157 | 27.08 | 16.03 | <0.0001 |
| CTAP | 16.67 | 79.17 | |||||
| CT | 38.89 | 5.33 | 0.021 | 27.08 | 16.94 | <0.0001 | |
| FDG-PET | 83.33 | 77.08 | |||||
| CTAP | 16.67 | 10.29 | 0.001 | 79.17 | 0.05 | 0.827 | |
| FDG-PET | 83.33 | 77.08 | |||||
| Peritoneum | CT | 47.83 | 10.00 | 0.002 | 38.10 | 12.00 | 0.001 |
| CTAP | 4.35 | 95.24 | |||||
| CT | 47.83 | 3.27 | 0.071 | 38.10 | 0.47 | 0.491 | |
| FDG-PET | 78.26 | 52.38 | |||||
| CTAP | 4.35 | 15.21 | <0.0001 | 95.24 | 7.36 | 0.007 | |
| FDG-PET | 78.26 | 52.38 | |||||
| Organ | CT | 50.00 | 2.00 | 0.157 | 33.33 | 6.40 | 0.011 |
| CTAP | 16.67 | 86.67 | |||||
| CT | 50.00 | 1.00 | 0.317 | 33.33 | 2.57 | 0.109 | |
| FDG-PET | 83.33 | 73.33 | |||||
| CTAP | 16.67 | 2.67 | 0.103 | 86.67 | 0.67 | 0.414 | |
| FDG-PET | 83.33 | 73.33 |
Pairwise tests for statistically significant differences between scan sensitivities were corrected for clustering using SAS PROC GENMOD. Unable to calculate estimates for size ≥5 cm. CT, computed tomography; CTAP, CT arterial portography; FDG-PET, [18F]2-fluoro-2-deoxyglucose positron emission tomography.
The detection of extrahepatic disease was directly related to the CRS (Figure 2). For CRS less than 3, the risk of extrahepatic disease is less than 25%. For a CRS of 4 or 5, the risk of extrahepatic disease is greater than 50%. Consequently, the yield of FDG-PET for detection of extrahepatic disease in patients with CRS <3 is 16%, while for CRS ≥3 it was 28%.
Hepatic disease
In these 125 patients, 399 liver lesions were found. Of these, 177 were confirmed. The sensitivity, specificity, negative predictive value, and positive predictive values are also calculated in Tables 3 and 4, according to tumor’s size and treatment with chemotherapy. Overall, FDG-PET had the lowest sensitivity, although specificity and the positive predictive value remained high, suggesting that if the liver lesion was hypermetabolic, it was highly likely to be cancer. The ROC curves in Figure 3 also suggest that FDG-PET was clearly inferior to cross-sectional imaging for detection of cancer in the liver.
Table 3
| Group | Scan | n | Test prevalence | (SE) | Pathology prevalence | (SE) | Sensitivity (%) | (SE) | Specificity (%) | (SE) | PPV | (SE) | NPV | (SE) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | CT | 399 | 47.37 | 3.63 | 44.36 | 3.92 | 78.53 | 3.71 | 77.48 | 4.77 | 73.54 | 5.28 | 81.91 | 3.75 |
| CTAP | 399 | 51.63 | 3.32 | 44.36 | 3.92 | 81.92 | 4.25 | 72.52 | 4.43 | 70.39 | 4.63 | 83.42 | 4.60 | |
| FDG-PET | 399 | 32.33 | 3.21 | 44.36 | 3.92 | 63.28 | 5.36 | 92.34 | 2.26 | 86.82 | 3.70 | 75.93 | 4.40 | |
| No Chemo | CT | 251 | 43.82 | 4.59 | 37.45 | 4.23 | 82.98 | 3.50 | 79.62 | 6.31 | 70.91 | 7.53 | 88.65 | 3.18 |
| CTAP | 251 | 51.00 | 4.18 | 37.45 | 4.23 | 87.23 | 4.13 | 70.70 | 6.05 | 64.06 | 6.18 | 90.24 | 4.01 | |
| FDG-PET | 251 | 32.27 | 3.87 | 37.45 | 4.23 | 77.66 | 4.83 | 94.90 | 2.05 | 90.12 | 3.93 | 87.65 | 3.01 | |
| Chemo | CT | 148 | 53.38 | 5.50 | 56.08 | 6.65 | 73.49 | 6.64 | 72.31 | 5.41 | 77.22 | 6.93 | 68.12 | 6.97 |
| CTAP | 148 | 52.70 | 5.23 | 56.08 | 6.65 | 75.90 | 7.70 | 76.92 | 4.09 | 80.77 | 5.16 | 71.43 | 9.39 | |
| FDG-PET | 148 | 32.43 | 5.59 | 56.08 | 6.65 | 46.99 | 8.23 | 86.15 | 5.75 | 81.25 | 7.28 | 56.00 | 8.52 | |
| ≤1 cm | CT | 138 | 39.86 | 6.03 | 28.26 | 5.45 | 58.97 | 9.35 | 67.68 | 7.06 | 41.82 | 9.25 | 80.72 | 5.59 |
| CTAP | 138 | 44.93 | 5.00 | 28.26 | 5.45 | 71.79 | 9.24 | 65.66 | 5.59 | 45.16 | 8.16 | 85.53 | 5.68 | |
| FDG-PET | 138 | 10.87 | 2.76 | 28.26 | 5.45 | 23.08 | 8.21 | 93.94 | 2.40 | 60.00 | 14.49 | 75.61 | 5.62 | |
| >1, <5 cm | CT | 228 | 57.89 | 3.99 | 59.65 | 4.41 | 85.29 | 2.94 | 82.61 | 4.36 | 87.88 | 3.26 | 79.17 | 4.52 |
| CTAP | 228 | 61.40 | 3.43 | 59.65 | 4.41 | 86.03 | 3.74 | 75.00 | 5.11 | 83.57 | 3.64 | 78.41 | 6.53 | |
| FDG-PET | 228 | 47.81 | 3.94 | 59.65 | 4.41 | 75.74 | 4.58 | 93.48 | 2.92 | 94.50 | 2.47 | 72.27 | 5.78 | |
| >5 cm | CT | 200 | 55.50 | 4.16 | 57.00 | 4.70 | 83.33 | 3.44 | 81.40 | 4.56 | 85.59 | 3.79 | 78.65 | 4.88 |
| CTAP | 200 | 59.00 | 3.75 | 57.00 | 4.70 | 83.33 | 4.46 | 73.26 | 5.49 | 80.51 | 4.25 | 76.83 | 6.97 | |
| FDG-PET | 200 | 44.00 | 4.16 | 57.00 | 4.70 | 71.93 | 5.29 | 93.02 | 3.20 | 93.18 | 3.11 | 71.43 | 6.03 |
CT, computed tomography; CTAP, CT arterial portography; FDG-PET, [18F]2-fluoro-2-deoxyglucose positron emission tomography; PPV, positive predictive value; NPV, negative predictive value; Chemo, chemotherapy.
Table 4
| Group | Scan | Sensitivity (%) | Chi-square (df =1) | P value | Specificity (%) | Chi-square (df =1) | P value |
|---|---|---|---|---|---|---|---|
| All | CT | 78.53 | 0.38 | 0.54 | 77.48 | 0.56 | 0.45 |
| CTAP | 81.92 | 72.52 | |||||
| CT | 78.53 | 7.67 | 0.006 | 77.48 | 7.51 | 0.006 | |
| FDG-PET | 63.28 | 92.34 | |||||
| CTAP | 81.92 | 6.08 | 0.01 | 72.52 | 10.08 | 0.002 | |
| FDG-PET | 63.28 | 92.34 | |||||
| No Chemo | CT | 82.98 | 0.89 | 0.35 | 79.62 | 0.97 | 0.32 |
| CTAP | 87.23 | 70.70 | |||||
| CT | 82.98 | 1 | 0.32 | 79.62 | 4.97 | 0.026 | |
| FDG-PET | 77.66 | 94.90 | |||||
| CTAP | 87.23 | 2.08 | 0.15 | 70.70 | 8.7 | 0.003 | |
| FDG-PET | 77.66 | 94.90 | |||||
| Chemo | CT | 73.49 | 0.05 | 0.82 | 72.31 | 0.69 | 0.40 |
| CTAP | 75.90 | 76.92 | |||||
| CT | 73.49 | 7.33 | 0.007 | 72.31 | 2.79 | 0.095 | |
| FDG-PET | 46.99 | 86.15 | |||||
| CTAP | 75.90 | 4.24 | 0.04 | 76.92 | 1.38 | 0.24 | |
| FDG-PET | 46.99 | 86.15 | |||||
| Size ≤1 cm | CT | 58.97 | 0.86 | 0.35 | 67.68 | 0.04 | 0.84 |
| CTAP | 71.79 | 65.66 | |||||
| CT | 58.97 | 7 | 0.008 | 67.68 | 10.9 | 0.001 | |
| FDG-PET | 23.08 | 93.94 | |||||
| CTAP | 71.79 | 6.56 | 0.01 | 65.66 | 12.65 | 0.0004 | |
| FDG-PET | 23.08 | 93.94 | |||||
| Size >1 cm | CT | 85.29 | 0.03 | 0.86 | 82.61 | 1.69 | 0.19 |
| CTAP | 86.03 | 75.00 | |||||
| CT | 85.29 | 3.6 | 0.058 | 82.61 | 5 | 0.025 | |
| FDG-PET | 75.74 | 93.48 | |||||
| CTAP | 86.03 | 2.97 | 0.09 | 75.00 | 9.32 | 0.002 | |
| FDG-PET | 75.74 | 93.48 |
CT, computed tomography; CTAP, CT arterial portography; FDG-PET, [18F]2-fluoro-2-deoxyglucose positron emission tomography.

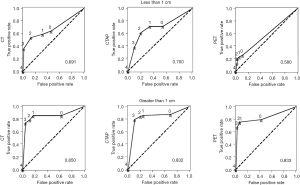
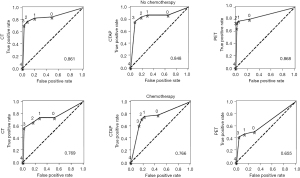
In particular, small tumors were poorly detected by FDG-PET. If a tumor was less than 1 cm in size, less than one-quarter of the time it was detectable by FDG-PET. In patients on chemotherapy, lesions were detectable by FDG-PET in less than half the cases. In fact, for lesions less than 1 cm in patients on chemotherapy, viable tumor was detectable by FDG-PET less than 10% of the time. This is also reflected in the ROC curves (Figures 4,5). In patients not on chemotherapy, the areas under the curve for the ROC curve (0.87) for FDG-PET was equivalent to CT or CTAP. When patients were on chemotherapy, the area dropped to 0.66. For large lesions (>1 cm), the area under the ROC curve was 0.83, equivalent to cross-sectional imaging. For lesions less than 1 cm, the area was 0.58, only a little better than flipping a coin.
Discussion
Accumulating experience with FDG-PET has suggested that this diagnostic modality may have clinical utility in the management of patients with hepatic colorectal metastases (10-14). Previous studies have suggested that FDG-PET is capable of identifying a subset of metastases missed by conventional diagnostic imaging modalities (11-23). There have been a number of small, mostly retrospective studies of the impact of FDG-PET on management of patients with hepatic colorectal metastases (11-23). Many other cross-sectional imaging modalities have also been developed and refined for staging of patients with hepatic colorectal metastases and for surgical planning. No prior study had fully compared utility of these exams according to site. No prior study performed independent blinded readings to allow assessment of effect of each test and combinations of tests. Employing a large prospective analysis of the impact of preoperative FDG-PET imaging on the clinical management of patients with hepatic colorectal metastases, we have observed that routine incorporation of FDG-PET into preoperative diagnostic evaluation can meaningfully alter the clinical management of approximately one-third of all patients presenting with potentially resectable hepatic colorectal metastases.
Patients eligible for enrollment into this study were referred for surgical therapy for potentially resectable hepatic colorectal metastases. By following these patients prospectively, we observed that 19% were determined to have unresectable disease based on preoperative imaging with CT, CTAP, and FDG-PET. In total, only 60% of patients referred for potential surgical therapy were able to undergo potentially curative operative interventions. Of the 101 patients who underwent operative intervention with curative intent, 26% were intraoperatively determined to have technically irresectable disease. The 74% resectability rate of patients undergoing operative intervention for hepatic colorectal metastases is not dissimilar from results seen in other series (7-9). The observation that 18% of patients had elements of their therapeutic management altered by virtue of findings seen on FDG-PET alone and another 13% had management altered partly by FDG-PET confirms previous conclusions that FDG-PET impacts the clinical management of patients with hepatic colorectal metastases (15-24). This observed impact of FDG-PET is somewhat lower than has been seen in other studies, likely because of the rigorous cross-sectional imaging performed, including CTAP (26). Indeed, in the present study, the impact of FDG-PET on clinical management was not in the detection of irresectability due to extent of intrahepatic disease.
It is also increasingly common that patients are subjected to neoadjuvant chemotherapy prior to attempted liver resection. We and others have previously demonstrated that the sensitivity of FDG-PET is significantly impaired by chemotherapy (11,31). The present study demonstrates that in a population where over half the patients are receiving chemotherapy, it is unlikely that FDG-PET will contribute significantly to staging of liver site of tumor in the setting of good cross-sectional imaging. CT and CTAP are better options for planning the liver-specific portion of the operation. Other have also found that FDG-PET is poor at detecting small viable tumors within the liver (32). In fact, the investigators in this prior study recommended against use of FDG-PET in the preoperative assessment based on the low predictive value of FDG-PET (13.3%) for viable tumor within the liver (32). We differ from their recommendation and believe a FDG-PET should be included in preoperative work-up because of its utility in finding extrahepatic unresectable disease. In previous studies the sensitivity of FDG-PET for detecting viable cancer has been reported to be as high as 94% (33,34). This is due to results interpreted on a per patient basis, and the utility of this test for detecting extrahepatic disease. Indeed, in the current study, we found the sensitivity of FDG-PET for extrahepatic disease to be 90%. For liver disease however, the results were much poorer: sensitivities of 63% overall, of 47% for patients on chemotherapy, and 23% for tumors less than 1 cm.
The impact of FDG-PET on clinical management was clearly most pronounced in the preoperative identification of irresectability due to extrahepatic disease (35). Of the 23 patients whose clinical management was altered by findings seen on FDG-PET alone, 16 were due to detection of extrahepatic disease. This observation is in agreement with previous studies that have suggesting notable impact of FDG-PET on the detection of occult disease outside the liver (14,19,20,22). Eight patients were spared an unnecessary laparotomy due to findings seen on FDG-PET alone. An additional 8 patients were spared an unnecessary partial hepatectomy due to findings seen on FDG-PET alone. It is conceivable that the diagnosis of extrahepatic disease might have been made at the time of operative exploration if FDG-PET imaging had not been performed. However, in each of these cases, the metastatic foci were small (either within nonenlarged lymph nodes or small peritoneal implants). Operative conduct was meaningfully altered in these cases, either by pursuing the area of FDG-PET abnormality with laparoscopy or by using a smaller incision. It is possible that in the future, FDG-PET-guided needle biopsies may allow additional patients to avoid nontherapeutic laparotomy. In addition, the impact of FDG-PET was not limited to the detection of unresectable extrahepatic disease; seven patients underwent potentially curative metastasectomy because FDG-PET alone disproved CT evidence of irresectable disease.
These data are also compatible with the recent randomized trial demonstrating no benefit for FDG-PET in the preoperative staging of patients (36). Our data demonstrates that FDG-PET as a preoperative test is good at detecting and verifying extrahepatic disease, while having little effect on staging of hepatic disease. In a population of patients that includes patients with low CRS and is well screened by cross sectional imaging and considered only to have disease confined to the liver, large numbers would need to be randomized to show utility of FDG-PET. From the Moulton data (36) and our current study, we would recommend FDG-PET in patients with high CRS or with cross-sectional imaging suspicious for extrahepatic disease.
Since FDG-PET is clearly able to accurately stage patients prior to hepatectomy, many patients are also being followed postoperatively (37), though little data justify the enormous expense of such surveillance. The current study is at least indirect evidence that such a follow-up strategy is not fruitful. In follow-up we are looking for small recurrences, usually in patients subjected to adjuvant chemotherapy. The hepatic site is also the most common first site of recurrence for patients after hepatectomy. Small hepatic lesions in patients undergoing chemotherapy are precisely the lesions with the lowest level of detection by FDG-PET.
There are limitations of current study. This study represents a prospective study comparing still the most used modalities in imaging a patient with liver metastases before surgery, specifically CT and FDG-PET. The data indicates that better imaging is needed. At the conception of this trial MRI was not included. Data has since emerged that in patients with small liver tumors, particularly if prior chemotherapy had led to hepatic toxicity and changes in background imaging characteristics, MRI is the best modality for preoperative staging of the liver and for surgical planning (38). The data in this prospective comparison of MRI to multidetector CT shows a sensitivity for CT at 74% (38), very much like our findings of 75% for CT in patients with prior chemotherapy. Our data would be supportive of the need for MRI as staging for the liver in patients who have undergone neoadjuvant chemotherapy.
These data support FDG-PET as an important test for preoperative staging of patients with hepatic colorectal metastases, altering treatment decisions in nearly one-third of patients. The high yield is due mainly to detection or confirmation of extrahepatic disease. It is particularly recommended in patients with high CRSs. It is not a good test for identification of small tumors in the liver.
Acknowledgments
Funding: This work was supported in part by grant NIH-R01CA/DK80982-01 from the National Institutes of Health and a grant the FAMRI.
Footnote
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-19-357/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-19-357/coif). YF serves as an unpaid editorial board member of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional ethics board of Memorial Sloan-Kettering Cancer Center and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66. [Crossref] [PubMed]
- Kato T, Yasui K, Hirai T, et al. Therapeutic results for hepatic metastasis of colorectal cancer with special reference to effectiveness of hepatectomy: analysis of prognostic factors for 763 cases recorded at 18 institutions. Dis Colon Rectum 2003;46:S22-31. [PubMed]
- Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg 2004;240:438-47; discussion 447-50. [Crossref] [PubMed]
- Stewart CL, Warner S, Ito K, et al. Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr Probl Surg 2018;55:330-79. [Crossref] [PubMed]
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271-80. [Crossref] [PubMed]
- Gibbs JF, Weber TK, Rodriguez-Bigas MA, et al. Intraoperative determinants of unresectability for patients with colorectal hepatic metastases. Cancer 1998;82:1244-9. [Crossref] [PubMed]
- Jarnagin WR, Fong Y, Ky A, et al. Liver resection for metastatic colorectal cancer: assessing the risk of occult irresectable disease. J Am Coll Surg 1999;188:33-42. [Crossref] [PubMed]
- Jarnagin WR, Conlon K, Bodniewicz J, et al. A clinical scoring system predicts the yield of diagnostic laparoscopy in patients with potentially resectable hepatic colorectal metastases. Cancer 2001;91:1121-8. [Crossref] [PubMed]
- Libutti SK, Alexander HR Jr, Choyke P, et al. A prospective study of 2-[18F] fluoro-2-deoxy-D-glucose/positron emission tomography scan, 99mTc-labeled arcitumomab (CEA-scan), and blind second-look laparotomy for detecting colon cancer recurrence in patients with increasing carcinoembryonic antigen levels. Ann Surg Oncol 2001;8:779-86. [Crossref] [PubMed]
- Akhurst T, Kates TJ, Mazumdar M, et al. Recent chemotherapy reduces the sensitivity of 18Ffluorodeoxyglucose positron emission tomography in the detection of colorectal metastases. J Clin Oncol 2005;23:8713-6. [Crossref] [PubMed]
- Rohren EM, Paulson EK, Hagge R, et al. The role of F-18 FDG positron emission tomography in preoperative assessment of the liver in patients being considered for curative resection of hepatic metastases from colorectal cancer. Clin Nucl Med 2002;27:550-5. [Crossref] [PubMed]
- Wiering B, Krabbe PF, Jager GJ, et al. The impact of fluor-18-deoxyglucose-positron emission tomography in the management of colorectal liver metastases. Cancer 2005;104:2658-70. [Crossref] [PubMed]
- Wiering B, Krabbe PF, Dekker HM, et al. The role of FDG-PET in the selection of patients with colorectal liver metastases. Ann Surg Oncol 2007;14:771-9. [Crossref] [PubMed]
- Fong Y, Saldinger PF, Akhurst T, et al. Utility of 18F-FDG positron emission tomography scanning on selection of patients for resection of hepatic colorectal metastases. Am J Surg 1999;178:282-7. [Crossref] [PubMed]
- Boykin KN, Zibari GB, Lilien DL, et al. The use of FDG-positron emission tomography for the evaluation of colorectal metastases of the liver. Am Surg 1999;65:1183-5. [PubMed]
- Zhuang H, Sinha P, Pourdehnad M, et al. The role of positron emission tomography with fluorine-18-deoxyglucose in identifying colorectal cancer metastases to liver. Nucl Med Commun 2000;21:793-8. [Crossref] [PubMed]
- Topal B, Flamen P, Aerts R, et al. Clinical value of whole-body emission tomography in potentially curable colorectal liver metastases. Eur J Surg Oncol 2001;27:175-9. [Crossref] [PubMed]
- Ruers TJ, Langenhoff BS, Neeleman N, et al. Value of positron emission tomography with F-18fluorodeoxyglucose in patients with colorectal liver metastases: a prospective study. J Clin Oncol 2002;20:388-95. [Crossref] [PubMed]
- Selzner M, Hany TF, Wildbrett P, et al. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg 2004;240:1027-34; discussion 1035-6. [Crossref] [PubMed]
- Schüssler-Fiorenza CM, Mahvi DM, Niederhuber J, et al. Clinical risk score correlates with yield of PET scan in patients with colorectal hepatic metastases. J Gastrointest Surg 2004;8:150-7; discussion 157-8. [Crossref] [PubMed]
- Truant S, Huglo D, Hebbar M, et al. Prospective evaluation of the impact of 18Ffluoro-2-deoxy-D-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg 2005;92:362-9. [Crossref] [PubMed]
- Joyce DL, Wahl RL, Patel PV, et al. Preoperative positron emission tomography to evaluate potentially resectable hepatic colorectal metastases. Arch Surg 2006;141:1220-6; discussion 1227. [Crossref] [PubMed]
- Wiering B, Ruers TJ, Krabbe PF, et al. Comparison of multiphase CT, FDG-PET and intra-operative ultrasound in patients with colorectal liver metastases selected for surgery. Ann Surg Oncol 2007;14:818-26. [Crossref] [PubMed]
- Ruers TJ, Wiering B, van der Sijp JR, et al. Improved selection of patients for hepatic surgery of colorectal liver metastases with (18)F-FDG PET: a randomized study. J Nucl Med 2009;50:1036-41. [Crossref] [PubMed]
- Schwartz L, Brody L, Brown K, et al. Prospective, blinded comparison of helical CT and CT arterial portography in the assessment of hepatic metastasis from colorectal carcinoma. World J Surg 2006;30:1892-9; discussion 1900-1. [Crossref] [PubMed]
- Cunningham JD, Fong Y, Shriver C, et al. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg 1994;129:1050-6. [Crossref] [PubMed]
- Ramos CD, Erdi YE, Gonen M, et al. FDG-PET standardized uptake values in normal anatomical structures using iterative reconstruction segmented attenuation correction and filtered back-projection. Eur J Nucl Med 2001;28:155-64. [Crossref] [PubMed]
- Obuchowski NA. Nonparametric analysis of clustered ROC curve data. Biometrics 1997;53:567-78. [Crossref] [PubMed]
- Gonen M. In: Analyzing ROC Curves Using SAS. SAS Press; 2007.
- Spatz J, Holl G, Sciuk J, et al. Neoadjuvant chemotherapy affects staging of colorectal liver metastasis--a comparison of PET, CT and intraoperative ultrasound. Int J Colorectal Dis 2011;26:165-71. [Crossref] [PubMed]
- Glazer ES, Beaty K, Abdalla EK, et al. Effectiveness of positron emission tomography for predicting chemotherapy response in colorectal cancer liver metastases. Arch Surg 2010;145:340-5; discussion 345. [Crossref] [PubMed]
- Floriani I, Torri V, Rulli E, et al. Performance of imaging modalities in diagnosis of liver metastases from colorectal cancer: a systematic review and meta-analysis. J Magn Reson Imaging 2010;31:19-31. [Crossref] [PubMed]
- Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology 2010;257:674-84. [Crossref] [PubMed]
- Lake ES, Wadhwani S, Subar D, et al. The influence of FDG PET-CT on the detection of extrahepatic disease in patients being considered for resection of colorectal liver metastasis. Ann R Coll Surg Engl 2014;96:211-5. [Crossref] [PubMed]
- Moulton CA, Gu CS, Law CH, et al. Effect of PET before liver resection on surgical management for colorectal adenocarcinoma metastases: a randomized clinical trial. JAMA 2014;311:1863-9. [Crossref] [PubMed]
- Jiménez Londoño GA, García Vicente AM, Sánchez Pérez V, et al. 18F-FDG PET/contrast enhanced CT in the standard surveillance of high risk colorectal cancer patients. Eur J Radiol 2014;83:2224-30. [Crossref] [PubMed]
- Granata V, Fusco R, de Lutio di Castelguidone E, et al. Diagnostic performance of gadoxetic acid-enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol 2019;19:129. [Crossref] [PubMed]



