
Highly variable biodistribution of 68Ga labeled somatostatin analogues 68Ga-DOTA-NOC and 68Ga-DOTA-TATE in neuroendocrine tumors: clinical implications for somatostatin receptor directed PET/CT
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of neoplasms that manifest throughout the body, particularly the gastrointestinal system and lungs (1-4). Despite their differences, these tumors share in common a high expression of somatostatin receptors (SSTRs), a property that has been harnessed by recent molecular imaging methods using SSTR analogs (5). SSTR-targeted probes have become widely utilized across routine clinical contexts for the management of NETs, and open new avenues for precision oncology when paired with a companion receptor-targeted radiotherapeutic agent (6-12). With the rapid adoption of SSTR-targeted positron emission tomography/computed tomography (PET/CT) imaging into the routine clinical management of NETs, it is necessary to evaluate the accuracy and reliability of interpreting the findings of SSTR-PET/CT imaging, particularly if the diagnostic intention extends beyond definition of sites of disease to an attempt to establish a quantitative measure of total tumor burden based on observed uptake values (13-19). SSTRs are found in both normal tissue (e.g., liver, spleen, pituitary, adrenal) and sites of disease; beyond detection of lesions, it is important to understand whether and how the magnitude of uptake observed in SSTR-targeted PET relates to disease state and therapeutic response (5,17,20-22).
The purpose of the present study is to assess the interscan variability and reproducibility of significant uptake variability (SUV) metrics in background tissue and NET deposits on 68Ga-DOTA-NOC and 68Ga-DOTA-TATE PET-CT imaging. We present the following article in accordance with the STROBE reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-554/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the Institutional Review Board at Indiana University in accordance with the Institutional Committee for the Protection of Human Subjects (IRB number: 1411891689). Inform consent for this retrospective study was waived.
Patients
PET/CT image in this study, we analyzed data from 81 patients who underwent routine whole-body 68Ga-DOTA-NOC and/or 68Ga-DOTA-TATE PET/CT (223 scans) to define the location and extent of disease between January 2014 to July 2019 at the Indiana University Health University Hospital, Indianapolis, USA. The 68Ga-DOTA-NOC imaging was performed as a clinical procedure under Expanded Access IND #117255. The 68Ga-DOTA-TATE studies employed the FDA-approved NetSpot™ product. All patients had undergone surgical resection of the primary NET lesion prior to the imaging studies, and had histopathologically proven grade 1–2 NETs. The clinical indications for PET/CT scanning were confirmed or suspected SST-expressing tumors [gastroenteropancreatic NET (48 patients), non-gastroenteropancreatic NET (originating in the lung or mediastinum; 8 patients), and NET of unknown primary (25 patients)]. This study also examined scan-to-scan variability of hepatic metastatic lesions in 21 patients with stable disease (58 PET/CT studies).
Image analysis
PET/CT image analysis was performed on all 81 patients using the MIM software (MIM Software Inc., Cleveland, OH, USA). We defined two groups of patients for the evaluation of target tissues. The first group consisted of all 81 patients (223 PET/CT studies). In this group, we evaluated the physiological 68Ga-DOTA-NOC/TATE uptake by normal target tissues as defined by the normal morphology on the CT part of the 68Ga-DOTA-NOC/TATE PET/CT scan. The normal biodistribution of 68Ga-DOTA-TATE is known to include the spleen, liver, pituitary, and adrenal gland (23). Volume of interests (VOIs) were placed within these organs in areas unaffected by disease. The following parameters were assessed for the normal organs: mean body weight corrected significant uptake variability (SUVmean) for the spleen and liver, and SUVmax for the pituitary and adrenal gland. Attenuation-corrected PET images and PET/CT images were analyzed. PET/CT fusion images were used to place the VOIs for SUVmax and SUVmean measurements. The second group consisted of 21 patients (58 PET/CT studies) who demonstrated stable disease with metastatic hepatic lesions. These studies were subsequently included in the analysis of tumor burden using SUVmean and SUVmax values derived from similarly applied VOI parameters.
Statistical analysis
Data were expressed as mean ± SD, using Microsoft Excel v. 2010. A two-sided t-test was used to determine the significance of differences between normal tissue and metastatic lesions. P values less than 0.05 were considered significant.
Results
Eighty-one patients were enrolled in this retrospective study, and all underwent routine whole-body 68Ga-DOTA-NOC and/or 68Ga-DOTA-TATE PET/CT. The mean age at first imaging was 57 years (range, 23–83 years). The female-to-male ratio was 1.08:1. The clinical indications for PET/CT scanning were confirmed or suspected SST-expressing malignancy (i.e., gastroenteropancreatic NET (48 patients), non-gastroenteropancreatic NET (originating in the lung or mediastinum; 8 patients), and NET of unknown primary (25 patients). Imaging was performed to detect tumor recurrence during post-therapy follow up (223 PET/CT studies). This study also examined interscan variability of hepatic metastatic lesions in 21 patients with stable disease (58 PET/CT studies) (Figures 1-4). The following parameters were assessed for the normal organs: mean body weight corrected SUVmean for the spleen and liver, and SUVmax for the pituitary and adrenal gland.
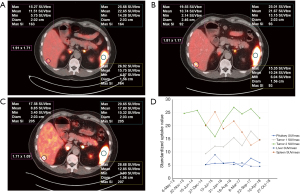
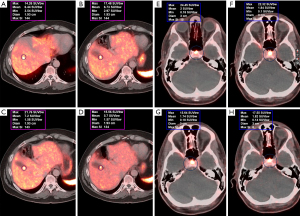
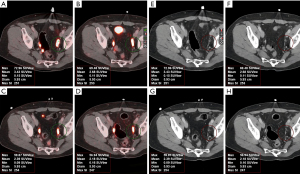
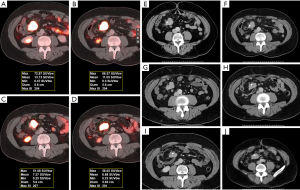
The median SUVmean was 16 for the spleen, 9.7 for the pituitary, 12.6 for the adrenal glands, and 4.8 for the liver (Table 1). The normal pituitary gland demonstrates focal homogenous uptake with SUVmax range of 4.5–23. The adrenal gland showed uptake with SUVmax range 4.1–29.4, which is more than two times greater than liver uptake (SUVmean range, 2.3–12.4). Highest normal physiological uptake was seen in the spleen (average SUVmean of 17.3, range of 5.4–34.4) (Figure 1). Metastatic hepatic lesions demonstrated greater variability of SUVmax than SUVmean (24.5±13.1 vs. 16.5±8.0). SUV measures were not significantly different when comparing intra-patient scans of 68Ga-DOTA-NOC and 68Ga-DOTA-TATE, with P values >0.05 for all normal tissue and metastatic hepatic lesions in patients with stable NETs.
Table 1
| Organs | Parameter | Patients | Minimum | Median | Maximum | Mean | StDev |
|---|---|---|---|---|---|---|---|
| Pituitary | SUVmax | 74 | 4.5 | 9.7 | 23 | 10.3 | 4 |
| Spleen | SUVmean | 59 | 5.4 | 16 | 34.4 | 17.3 | 6.7 |
| Liver | SUVmean | 81 | 2.3 | 4.8 | 12.4 | 5 | 2 |
| Adrenal glands | SUVmax | 73 | 4.1 | 12.6 | 29.4 | 13 | 5.6 |
| Tumor burden | SUVmean | 21 | 3.7 | 13 | 32.7 | 16.5 | 8 |
| SUVmax | 21 | 4.2 | 20.2 | 55.3 | 24.5 | 13.1 |
NET, neuroendocrine tumor; SUV, significant uptake variability; StDev, standard deviation.
Discussion
SSTR-targeted PET/CT imaging has risen to the forefront for NET detection and management (7-9,16,18), yet the variability of SUV as a semiquantitative measure of disease detection and tumor response to treatment has not been fully explored. The reported studies were performed prior to routine clinical availability of 177Lu-DOTA-TATE for SSTR-targeted radiotherapy, so the data represent the stability of imaging findings in patients undergoing only standard-of-care medical management. For both 68Ga-DOTA-NOC and 68Ga-DOTA-TATE this study demonstrated significant variability in SUV for both normal tissue and metastatic hepatic lesions in patients with stable NETs. In this study, we assess the reproducibility and scan-to-scan variance observed in serial clinical 68Ga-DOTA-NOC and 68Ga-DOTA-TATE PET/CT scans to monitor disease in patients with NETs undergoing only medical management of their disease.
Total injected mass of DOTA-NOC or DOTA-TATE, which can compete with the radiopharmaceutical for SSTR binding, will intrinsically vary with the quantity of 68Ga used in the synthesis (thus, varying with the age of the Ga-68 generator), and with the elapsed time between radiopharmaceutical compounding and administration. For the reported cases, those effects were intrinsically minimized by in-house compounding, and immediate use, of the radiopharmaceuticals. We observed no changes in image appearance that could be correlated with the injected ligand mass. Multiple intra-patient scans demonstrated no significant difference in scan-to-scan variability between 68Ga-DOTA-NOC and 68Ga-DOTA-TATE, with improved reproducibility with DOTA-TATE.
68Ga-SSTR PET/CT offers greater reliability for NET detection than previously utilized radionuclide imaging techniques, including 18F-FDG PET, 18F-DOPA PET, and bone scintigraphy (14,15,20,24-29). Given the significant role 68Ga-SSTR PET/CT plays in the clinical management of NETs, it becomes increasingly important to have a clear understanding of the role that quantified measures of uptake should play in assessing disease activity and prognosis. While quite sensitive for detection of the location of disease, in this study we observe dramatic variability in SSTR-targeted uptake in both normal tissue and tumor lesions, underscoring the limitations of SUV as a semiquantitative measure of NET activity.
SUV variability may be influenced by various factors including image reconstruction techniques and intrinsic scanner properties (20,30,31). Notably, variability of SSTR uptake has been partly attributed to the “tumor sink effect”, a phenomenon in which diminished activity in background organs (e.g., spleen) is correlated with increased tumor burden (32,33). Moreover, because SSTR expression is greatest in well-differentiated tumors, it is possible that undifferentiated tumor clones may become SSTR-negative; thus, 68Ga-SSA uptake values serve as an indirect measure of tumor differentiation in which greater uptake is correlated with improved prognosis (18,34). Indeed, the current study demonstrates that SUVmax of metastatic hepatic lesions in stable disease are greater than averaged normal tissue uptake values. Future studies may further evaluate the prognostic implications in NETs across stable and progressive or changing disease states (35).
The majority of these patients were undergoing treatment with sustained release octreotide to ameliorate NET symptoms. The Sandostatin® LAR Depot package insert indicates that after three doses the serum reaches a steady state concentration of octreotide. So, for patients on maintenance with that therapy, restriction on PET/CT timing should be unnecessary. Nevertheless, for these studies the SSTR-PET was timed to occur in the week leading up to repeat clinical dosing with sustained-release octreotide. It is not known whether individual patients can undergo temporal variations in serum octreotide levels, or the level of anti-octreotide antibodies, that might contribute to the variations seen between scans in the magnitude of 68Ga-DOTA-NOC or 68Ga-DOTA-TATE PET/CT when other clinical evidence (e/g., lesion size on CT) indicates disease stability.
Combined imaging using functional (e.g., 68Ga-DOTA-NOC or 68Ga-DOTA-TATE) and morphological features (e.g., CT/MRI) may also synergistically improve detection of NETs and measurement of therapeutic response (13-19). For example, 18F-FDG PET/CT is a well-established imaging modality that guides management of many oncologic diseases, but has had limited value in evaluating NETs because of their slow growth, reduced metabolic activity, and decreased rates of differentiation. Recent studies, however, have suggested the utility of 18F-FDG in detecting dedifferentiated but morphologically growing lesions with little to no expression of SSTR, which have conflicting 68Ga-DOTATATE findings (15,18,21,36).
In summary, our study demonstrated significant variability in SUV in both normal tissues as well as in primary and metastatic hepatic lesions in patients with stable NETs. The data support the need of incorporation of more reliable quantitative measure in the context of clinical decisions making. Caution is warranted if 68Ga-SSTR PET SUV is used alone as a measure of change in disease status.
Acknowledgments
Presented in part at the Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI), June 11–15, 2021.
Funding: Monica Cheng was the recipient of the Radiological Society of North America (RSNA) Research & Education Foundation Research Medical Student Grant (2020–2021).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-554/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-554/dss
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-554/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The research was approved by the Institutional Review Board at Indiana University in accordance with the Institutional Committee for the Protection of Human Subjects (IRB number: 1411891689). Inform consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Caplin ME, Ratnayake GM. Diagnostic and therapeutic advances in neuroendocrine tumours. Nat Rev Endocrinol 2021;17:81-2. [Crossref] [PubMed]
- Chauhan A, Kohn E, Del Rivero J. Neuroendocrine Tumors-Less Well Known, Often Misunderstood, and Rapidly Growing in Incidence. JAMA Oncol 2020;6:21-2. [Crossref] [PubMed]
- Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin 2018;68:471-87. [Crossref] [PubMed]
- Rindi G, Wiedenmann B. Neuroendocrine neoplasia goes molecular - time for a change. Nat Rev Clin Oncol 2019;16:149-50. [Crossref] [PubMed]
- Kunikowska J, Królicki L, Pawlak D, et al. Semiquantitative analysis and characterization of physiological biodistribution of (68)Ga-DOTA-TATE PET/CT. Clin Nucl Med 2012;37:1052-7. [Crossref] [PubMed]
- Strosberg J, El-Haddad G, Wolin E, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med 2017;376:125-35. [Crossref] [PubMed]
- Hope TA, Bodei L, Chan JA, et al. NANETS/SNMMI Consensus Statement on Patient Selection and Appropriate Use of 177Lu-DOTATATE Peptide Receptor Radionuclide Therapy. J Nucl Med 2020;61:222-7. [Crossref] [PubMed]
- Frilling A, Clift AK. Combining radiolabelled therapies for neuroendocrine neoplasms. Nat Rev Endocrinol 2020;16:347-8. [Crossref] [PubMed]
- Rindi G, Wiedenmann B. Neuroendocrine neoplasia of the gastrointestinal tract revisited: towards precision medicine. Nat Rev Endocrinol 2020;16:590-607. [Crossref] [PubMed]
- Kaderli RM, Spanjol M, Kollár A, et al. Therapeutic Options for Neuroendocrine Tumors: A Systematic Review and Network Meta-analysis. JAMA Oncol 2019;5:480-9. [Crossref] [PubMed]
- Basu S, Parghane RV. Peptide Receptor Radionuclide Therapy of Neuroendocrine Tumors. Semin Nucl Med 2020;50:447-64. [Crossref] [PubMed]
- Ambrosini V, Fanti S. Radioguided surgery with 68Ga-DOTATATE for patients with neuroendocrine tumors. Hepatobiliary Surg Nutr 2020;9:67-9. [Crossref] [PubMed]
- Hope TA, Bergsland EK, Bozkurt MF, et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J Nucl Med 2018;59:66-74. [Crossref] [PubMed]
- Sanli Y, Garg I, Kandathil A, et al. Neuroendocrine Tumor Diagnosis and Management: 68Ga-DOTATATE PET/CT. AJR Am J Roentgenol 2018;211:267-77. [Crossref] [PubMed]
- Tirosh A, Kebebew E. The utility of 68Ga-DOTATATE positron-emission tomography/computed tomography in the diagnosis, management, follow-up and prognosis of neuroendocrine tumors. Future Oncol 2018;14:111-22. [Crossref] [PubMed]
- Werner RA, Hänscheid H, Leal JP, et al. Impact of Tumor Burden on Quantitative 68Ga DOTATOC Biodistribution. Mol Imaging Biol 2019;21:790-8. [Crossref] [PubMed]
- Graham MM, Gu X, Ginader T, et al. 68Ga-DOTATOC Imaging of Neuroendocrine Tumors: A Systematic Review and Metaanalysis. J Nucl Med 2017;58:1452-8. [Crossref] [PubMed]
- Bodei L, Ambrosini V, Herrmann K, et al. Current Concepts in 68Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J Nucl Med 2017;58:1718-26. [Crossref] [PubMed]
- Werner RA, Bluemel C, Allen-Auerbach MS, et al. 68Gallium- and 90Yttrium-/ 177Lutetium: "theranostic twins" for diagnosis and treatment of NETs. Ann Nucl Med 2015;29:1-7. [Crossref] [PubMed]
- Cox CPW, Segbers M, Graven LH, et al. Standardized image quality for 68Ga-DOTA-TATE PET/CT. EJNMMI Res 2020;10:27. [Crossref] [PubMed]
- Bodei L, Schöder H, Baum RP, et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol 2020;21:e431-43. [Crossref] [PubMed]
- Shah MH, Goldner WS, Halfdanarson TR, et al. NCCN Guidelines Insights: Neuroendocrine and Adrenal Tumors, Version 2.2018. J Natl Compr Canc Netw 2018;16:693-702. [Crossref] [PubMed]
- Moradi F, Jamali M, Barkhodari A, et al. Spectrum of 68Ga-DOTA TATE Uptake in Patients With Neuroendocrine Tumors. Clin Nucl Med 2016;41:e281-7. [Crossref] [PubMed]
- Waldmann CM, Stuparu AD, van Dam RM, et al. The Search for an Alternative to [68Ga]Ga-DOTA-TATE in Neuroendocrine Tumor Theranostics: Current State of 18F-labeled Somatostatin Analog Development. Theranostics 2019;9:1336-47. [Crossref] [PubMed]
- Putzer D, Gabriel M, Henninger B, et al. Bone metastases in patients with neuroendocrine tumor: 68Ga-DOTA-Tyr3-octreotide PET in comparison to CT and bone scintigraphy. J Nucl Med 2009;50:1214-21. [Crossref] [PubMed]
- Adams S, Baum R, Rink T, et al. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med 1998;25:79-83. [Crossref] [PubMed]
- Putzer D, Gabriel M, Kendler D, et al. Comparison of (68)Ga-DOTA-Tyr(3)-octreotide and (18)F-fluoro-L-dihydroxyphenylalanine positron emission tomography in neuroendocrine tumor patients. Q J Nucl Med Mol Imaging 2010;54:68-75. [PubMed]
- Chu KKW, Chan ACY, Ma KW, et al. Role of C11-FDG dual-tracer PET-CT scan in metastatic screening of hepatocellular carcinoma-a cost-effectiveness analysis. Hepatobiliary Surg Nutr 2021;10:301-7. [Crossref] [PubMed]
- Armstrong EA, Beal EW, Shah M, et al. Radiographic characteristics of neuroendocrine liver metastases do not predict clinical outcomes following liver resection. Hepatobiliary Surg Nutr 2020;9:1-12. [Crossref] [PubMed]
- Boellaard R, Krak NC, Hoekstra OS, et al. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J Nucl Med 2004;45:1519-27. [PubMed]
- Jaskowiak CJ, Bianco JA, Perlman SB, et al. Influence of reconstruction iterations on 18F-FDG PET/CT standardized uptake values. J Nucl Med 2005;46:424-8. [PubMed]
- Kroiss A, Putzer D, Decristoforo C, et al. 68Ga-DOTA-TOC uptake in neuroendocrine tumour and healthy tissue: differentiation of physiological uptake and pathological processes in PET/CT. Eur J Nucl Med Mol Imaging 2013;40:514-23. [Crossref] [PubMed]
- Beauregard JM, Hofman MS, Kong G, et al. The tumour sink effect on the biodistribution of 68Ga-DOTA-octreotate: implications for peptide receptor radionuclide therapy. Eur J Nucl Med Mol Imaging 2012;39:50-6. [Crossref] [PubMed]
- Campana D, Ambrosini V, Pezzilli R, et al. Standardized uptake values of (68)Ga-DOTANOC PET: a promising prognostic tool in neuroendocrine tumors. J Nucl Med 2010;51:353-9. [Crossref] [PubMed]
- Xiang JX, Lv Y, Zhang XF. Surgical treatment for neuroendocrine liver metastasis: moving ahead in controversy. Hepatobiliary Surg Nutr 2021;10:868-71. [Crossref] [PubMed]
- Garcia-Carbonero R, Sorbye H, Baudin E, et al. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology 2016;103:186-94. [Crossref] [PubMed]


