
Transcatheter arterial embolization-salvaged ALPPS, a novel ALPPS procedure especially for patients with hepatocellular carcinoma and severe fibrosis/cirrhosis
Introduction
Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) is an innovation that provides patients with an opportunity to undergo curative liver resection for liver tumors which are initially unresectable (1,2). It has been increasingly used in liver surgery despite its initial controversies. Its use in patients with hepatocellular carcinoma (HCC) and chronic liver diseases has also been reported (3-8). ALPPS in selected HCC patients with severe fibrosis/cirrhosis has been accepted to be feasible with acceptable short-term results and it can induce adequate liver hypertrophy in these patients with a similar safety profile as in liver tumors arising from a normal liver background, such as colorectal cancer liver metastasis (CRLM) (3,5-8). However, hypertrophy of future liver remnant (FLR) is negatively correlated with severity of fibrosis and cirrhosis (3,8). Patients with severe fibrosis or cirrhosis are often associated with limited growth of FLR, leading to failure of ALPPS. Data from the International ALPPS Registry on a cohort of 35 patients showed that the mean kinetic growth rates (KGRs) of patients with cirrhosis or METAVIR grade 3 fibrosis were only 1.52±0.40 and 2.98±0.65 mL/day, respectively, whereas the KGRs of patients without fibrosis/cirrhosis or with grade 1 fibrosis were 30.94±10.95 and 12.34±4.38 mL/day, respectively (3). Our previous study on 45 HCC patients also demonstrated that the median KGRs in patients with cirrhosis or grade-3 fibrosis were 9.6 and 19.8 mL/day respectively, while the KGR in patients with a normal liver (grade 0) was 50.1 mL/day (8). Moreover, an attenuated increase in FLR has also been observed in the second week of ALPPS in patients with fibrosis or cirrhosis to be considerably lower than the KGR in the first week after ALPPS stage-1. In our previously reported study, three patients failed to reach sufficient increase in FLR volumes even up to 3–4 weeks after ALPPS stage-1. Two of them were converted to receive other palliative treatments. Another patient developed posthepatectomy liver failure (PHLF) and died of liver failure (8). Therefore, there are major challenges for ALPPS in treating patients with HCC and severe fibrosis/cirrhosis. Further measures to improve FLR hypertrophy in these patients by using modifications in the conventional ALPPS should be studied.
In 2017, a new procedure named TAE-salvaged ALPPS in treating one patient with a huge HCC and grade-3 fibrosis who failed to achieve sufficient FLR increase in 2 weeks after ALPPS stage-1 was reported by us (9). To slow down tumor progression, transcatheter arterial embolization (TAE) was used. Hepatic arteriography before embolization showed arterial steal of blood flow to FLR by the vascular tumor. TAE, in addition to decreasing arterial blood supply to the tumor to slow down tumor progression, also significantly improved arterial supply to the FLR. A fast increase in FLR volume was unexpectedly observed. The failed conventional ALPPS was salvaged and the tumor was successfully resected 1 week after TAE (9). These results suggested that this new ALPPS procedure can be used as a salvage procedure for patients with HCC and severe fibrosis/cirrhosis who failed conventional ALLPS stage-1. Nine consecutive HCC patients who presented with attenuated FLR hypertrophy after conventional ALPPS stage-1 were treated with the new ALPPS procedure afterwards. All the failed operations were salvaged, and curative liver resections were achieved. The purpose of this retrospective cohort study is to introduce the short-term outcomes of the new TAE-salvaged ALPPS procedure in the 10 HCC patients with cirrhosis or severe fibrosis, and to determine the feasibility and safety of the new procedure in these patients who were previously considered as unresectable. We present the following article in accordance with the STROCSS reporting checklist (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-466/rc).
Methods
Patients
Data on consecutive HCC patients who underwent TAE-salvaged ALPPS at Fudan University Zhongshan Hospital between November 2016 and June 2020 were retrospectively analyzed. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhongshan Hospital Fudan University. (No. B2021-879) and informed consent was taken from all individual participants. All patients were histopathologically studied for the degree of fibrosis or cirrhosis using the METAVIR scoring (10). Preoperative positron emission tomography/computed tomography (PET/CT), volumetric computed tomography (VCT), and magnetic resonance imaging (MRI) were routinely performed to exclude extrahepatic metastases and assess FLR volumes. All patients met the following inclusion criteria: a single large or multiple HCC nodules located in the hemiliver which required right hemihepatectomy or extended right hemihepatectomy; no tumors in the FLR; preoperative transient elastography of liver stiffness indicating cirrhosis/fibrosis; FLR/estimated standard liver volume (SLV) <35% (fibrosis) or 40% (cirrhosis); Child-Pugh class A; indocyanine green retention rate at 15 minutes (ICG-R15) ≤12%; Eastern Cooperative Oncology Group (ECOG) score 0–1; and Platelet count >50×109/L. Presence of tumor thrombosis in the right portal vein or right and/or middle hepatic veins was not a contraindication for the procedure. Patients who met the antiviral therapy criteria of the Asian Pacific Association for the Study of the Liver (APASL) received Entecavir (0.5 mg) daily, and Adefovir (10 mg) daily was added to patients who were resistant to Entecavir. Patients were stratified according to the Barcelona Clinic Liver Cancer (BCLC) staging and the China Liver Cancer (CNLC) staging Systems (11,12). Preoperative liver stiffness was measured by transient elastography and evaluated as reported by Zhuang et al. (13). Liver resection and anatomy were defined according to the Couinaud’s segmentation of the liver and the Brisbane 2000 Terminology of Liver Anatomy and Resections. Gradings of PHLF were defined according to the International Study Group of Liver Surgery (ISGLS) classification (14). Postoperative complications (POC) were defined according to the Clavien-Dindo criteria, and grades IIIb, IV, and V were considered as severe complications (15). Ascites was defined as postoperative daily abdominal drainage exceeding 10 mL/kg of body weight (16); postoperative hemorrhage was defined as a drop in hemoglobin level >3 g/dL compared with the baseline level, and/or any postoperative transfusion of packed red blood cells (RBC) for a falling hemoglobin (17); biliary leakage was defined by a bilirubin concentration in the drainage fluid with at least three times greater than that in serum concentration on or after postoperative days 3 (18). Postoperative mortality was measured within 90 days of surgery.
Surgical procedures
The TAE-salvaged ALPPS consists of 3 procedural steps: conventional ALPPS stage-1, TAE, and then conventional ALPPS stage-2 (Figure 1).
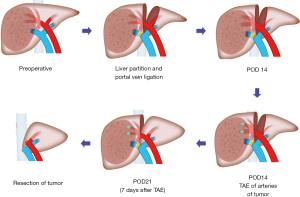
Step 1: ALPPS stage-1
The ALPPS stage-1 has been described in our previous studies (8,9).
Step 2: TAE of arteries supplying the tumor
TAE of the arteries supplying the tumor has been previously reported (19). Briefly, using the Seldinger technique, a Fr 4 hepatic catheter was inserted through a femoral artery into the hepatic artery. The tip of the catheter was advanced into the tumor-feeding artery for selective embolization of the tumor. Around 5 to 7 mL of lipiodol ultrafluid were slowly injected under fluoroscopy, followed by injection of 100–500 µm of microspheres and 350–560 µm of gelatin sponge particles. Only the arteries of tumor were selectively embolized, the arteries of non-tumor area were kept intact.
Step 3: ALPPS stage-2
ALPPS stage-2 was performed using the technique described in our previous studies (8,9).
Postoperative management and follow-up
After hospital discharge, patients were followed-up once every month for 3 months, and then once every 3 monthly. Adjuvant transcatheter arterial chemoembolization (TACE) was given in postoperative week 4 as previously described (20). Tumor recurrence was assessed by radiological (MRI, CT and PET/CT scan) and laboratory investigations.
Volumetric assessment
Liver volumes were measured by the IQQA-Liver system (EDDA Tech., Princeton, NJ, USA), and 3D reconstruction was performed using the Yorktal 3D reconstruction system as previously described (8,9). The estimated SLV was calculated using the Urata formula (21). Volumetric assessments were repeated in 1 and 2 weeks after ALPPS stage-1, and one week after TAE. The increase in FLR volume was evaluated until it met the criteria required of a FLR/SLV ratio ≥35% (fibrosis) or ≥40% (cirrhosis) for ALPPS stage-2 in patients with HCC and fibrosis/cirrhosis. Increase in FLR volume (the difference between FLR after ALPPS stage-1 and baseline FLR) and the daily increase in FLR volume (the KGRs) were calculated. The KGR was also a factor to consider whether to proceed to ALPPS stage-2, which was decided in some patients who had a high KGR but a FLR volume which was approaching the limit as defined by the pre-determined criteria.
Endpoints
The primary endpoint was treatment efficacy of the TAE-salvaged ALPPS (the R0 resection rate and hypertrophy of FLR), and treatment safety (the morbidity and 90-day mortality rates). Feasibility was studied by assessing the R0 resection rate or failure to proceed with ALPPS stage-2. Changes in liver parameters and other short-term outcomes were also analyzed.
Statistical analysis
Descriptive statistics were performed for demographic and clinical outcome parameters. Metric data were expressed as median with ranges for non-normally distributed data or mean ± standard deviation for normally distributed data. The SPSS software (SPSS version 20, IBM, USA) was used for statistical evaluations.
Results
Ten patients with HCC who underwent TAE-salvaged ALPPS were included in this study (Table 1). The median age was 56 (range, 43–66) years and the male to female ratio was 9:1. Nine patients had a single large tumor in right liver, with a median diameter of 14.0 (range, 5.2–17.0) cm. The remaining patient had multiple tumors (diameter of one tumor 14 cm and two satellite foci 2.0 and 3.0 cm, with the total tumor size of 19.0 cm). Two patients had large-vessel involvement (1 patient had right anterior portal vein and middle hepatic vein invasion; another patient had middle and right hepatic vein invasion). Seven patients (70.0%) were classified as BCLC Stage A, 1 patient (10.0%) as Stage B, and 2 patients (20.0%) as Stage C. The CNLC Staging System classified 7, 1 and 2 patients as Ib, IIa and IIIa stages, respectively. Two patients had TACE treatment one month before TAE-salvaged ALPPS. One patient had associated diabetes mellitus whilst 2 patients had hypertension. The remaining 7 patients had no associated comorbidities. All patients were in Child-Pugh class A. The median preoperative model for end-stage liver disease (MELD) score was 3.84 (range, 1.30–10.10). The median ICG-R15 was 5.1 (2.0–12.0). Six patients were histopathologically diagnosed as cirrhosis (METAVIR grade 4), 1 patient as METAVIR grade 3 severe fibrosis and 3 patients as METAVIR grade 2 fibrosis. The preoperative liver transient elastography of liver stiffness was 13.2 (5.1–16.4).
Table 1
| Patient characteristics | Data |
|---|---|
| Age, year, median (range) | 56 (43–66) |
| Gender, male/female, n (%) | 9/1 (90.0/10.0) |
| Single tumor (n=9) | |
| Diameter of tumor, cm, median (range) | 14.0 (5.2–17.0) |
| Multiple tumors (n=1) | |
| Number of lesions | 3 |
| Sum of diameters, cm | 19 |
| BCLC staging, n (%) | |
| A | 7 (70.0) |
| B | 1 (10.0) |
| C | 2 (20.0) |
| CNLC staging, n (%) | |
| Ib | 7 (70.0) |
| IIa | 1 (10.0) |
| IIIa | 2 (20.0) |
| METAVIR grade of liver fibrosis, n (%) | |
| Grade 2 (fibrosis) | 3 (30.0) |
| Grade 3 (fibrosis) | 1 (10.0) |
| Grade 4 (cirrhosis) | 6 (60.0) |
| Transient elastography, median (range) | 13.2 (5.1–16.4) |
| HBV-DNA, copies, median (range) | 17,100 (308–320,000) |
| Child-Pugh score, median (range) | 5 (5–5) |
| ICG-R15, median (range) | 5.1 (2.0–12.0) |
| MELD score, median (range) | 3.84 (1.30–10.10) |
BCLC, Barcelona Clinic Liver Cancer; CNLC staging, China Liver Cancer staging; HBV, hepatitis B virus; ICG-R15, indocyanine green 15-min retention test; MELD, model for end-stage liver disease.
R0 resection was achieved in all patients (100.0%) after TAE-salvaged ALPPS. The ALPPS stage-2 consisted of 9 extended right hemihepatectomy and 1 right hemihepatectomy. The ALPPS stage-1 was performed by open and laparoscopic operations in 8 and 2 patients respectively. Conventional complete liver parenchymal transections were performed in all these patients. Data on intraoperative operative times, blood loss and blood transfusions are shown in Table 2. No blood transfusions were required during ALPPS stage-1 in all these patients. However, in ALPPS stage-2, RBC were transfused in 4 patients for intraoperative blood loss, with 4 units in each patient.
Table 2
| Median (range) | |
|---|---|
| ALPPS stage-1 | |
| Operative time (min) | 225 (130–390) |
| Blood loss (mL) | 150 (100–500) |
| RBC transfusion (U) | 0 |
| ALPPS stage-2 | |
| Operative time (min) | 175 (114–345) |
| Blood loss (mL) | 400 (200–1,500) |
| RBC transfusion (U) | 0 (0–4) |
| Total parenchymal transection (%) | 100.0 |
| R0 resection of tumor (%) | 100.0 (10/10) |
| Type of operation (three steps), n (%) | |
| Open ALPPS Stage-1; TAE; Open ALPPS Stage-2 | 8 (80.0) |
| Lapa ALPPS Stage-1; TAE; Lapa ALPPS Stage-2 | 1 (10.0) |
| Lapa ALPPS Stage-1; TAE; Open ALPPS Stage-2 | 1 (10.0) |
| Extent of resection | |
| Right hemihepatectomy, n (%) | 1 (10.0) |
| Extended right hemihepatectomy, n (%) | 9 (90.0) |
| Repeat laparotomy rate (%) | 0 |
| Postoperative hospital stay days | 14 (1–21) |
ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; RBC, red blood cell; TAE, transcatheter arterial embolization; Lapa, laparoscopic.
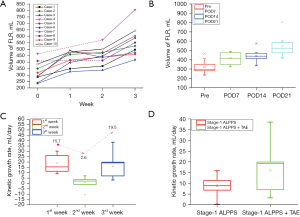
Data on preoperative baseline FLR volumes and postoperative increases in FLR volumes are shown in Table 3. The median preoperative FLR/SLV ratio of the 10 patients was 24.5% (range, 19.7–36.3%), and the median volume of FLR was 306.9 (range, 236.6–467.0) mL. A fast increase in FLR volume was observed in the first week after ALPPS stage-1. However, the increase in FLR volume became significantly attenuated in the second week (median KGR were 15.7 and 2.6 mL/day at weeks 1 and 2 after ALPPS stage-1, respectively) (Figure 2). At postoperative week 2 (POW2) after ALPPS stage-1, sufficient increase in FLR volume was not achieved in all these patients. However, increase in tumor volumes was observed in 8 patients (80.0%). The median volume of tumor at POW2 increased from preoperative 800.5 to 832.6 mL. Of the 8 patients who were AFP positive, 6 had good AFP follow-up data. The median AFP levels increased in all these 6 patients on postoperative day 10 (POD 10) (range, 8–13) (Figure S1). The increase in AFP levels and tumor volumes indicated significant tumor progression to be detectable in approximately 2 weeks after ALPPS stage-1. After TAE of the supplying artery to the tumor, significant increase was detected in FLR volume (median absolute and relative KGR were 19.5 mL/day and 4.1% after TAE, respectively). The FLR quickly hypertrophied to a sufficient volume for ALPPS stage-2 which was then successfully carried out in all these patients. The AFP levels were significantly decreased after TAE (Figure S1). The median increase in FLR volume between the two stages of ALPPS was 69.7% (range, 34.4–143.9%). The absolute and relative KGRs were 9.9 (range, 7.1–17.3) mL/day and 3.4% per day (range, 1.9–7.2%), respectively. The median waiting time for ALPPS stage-2 was 21 days (range, 18–24 days). TAE significantly accelerated the increase in FLR volume. The median absolute and relative KGRs in 2 weeks after ALPPS stage-1 were 8.9 mL/day and 1.9% before TAE, compared with those in the 3rd week after TAE of 19.5 mL/day and 3.2%, respectively (Figure 2D).
Table 3
| Variable | Median (range) |
|---|---|
| SLV (urata formula), mL | 1,285.1 (1,190.4–1,400.9) |
| FLR, mL | |
| Preoperative | 306.9 (236.6–467.0) |
| POW1 | 438.5 (328.0–490.0) |
| POW2 | 443.7 (339.6–574.5) |
| POW3 | 539.1 (419.9–806.0) |
| FLR/SLV, % | |
| Preoperative | 24.5 (19.7–36.3) |
| POW1 | 33.4 (27.3–38.1) |
| POW2 | 34.1 (28.2–44.7) |
| POW3 | 42.7 (34.9–62.7) |
| Absolute KGR, mL/day | |
| First week (after ALPPS stage-1) | 15.7 (9.2–30.3) |
| Second week (after ALPPS stage-1) | 2.6 (−10.4–7.1) |
| Third week (after TAE) | 19.5 (3.3–38.6) |
| Relative KGR, % | |
| First week (after ALPPS stage-1) | 4.6 (3.2–10.9) |
| Second week (after ALPPS stage-1) | 0.6 (–2.1–1.8) |
| Third week (after TAE) | 4.1 (0.7–6.7) |
FLR, future liver remnant; SLV, standard liver volume; POW, postoperative week; KGR, kinetic growth rate (daily FLR increase); ALPPS, associating liver partition and portal vein ligation for staged hepatectomy; TAE, transcatheter arterial embolization.
No patients required reoperation. The overall morbidity rates were 50.0% (5/10), 20.0% (2/10) and 70.0% (7/10) after ALPPS stage-1, TAE and ALPPS stage-2, respectively. There were 26 POCs (7, 4, and 15 developed after ALPPS stage-1, TAE and ALPPS stage-2, respectively) with 1 patient having 8 POCs (Tables S1,S2). Clavien-Dindo grade I and II POCs were common, while severe POCs (>grade IIIa) were uncommon, with no one after ALPPS stage-1 and TAE, and only 1 after ALPPS stage-2. All patients presented with fever after ALPPS stage-1 and after TAE despite antibiotics were routinely used (Figure S2A). Adequate hydration and diuretics were routinely used and tumor lysis was adequately managed, except in one patient (case-4). This patient received TACE one month before. He developed severe tumor lysis with subsequent renal dysfunction after ALPPS stage-1 and TAE, presenting with the typical tumor lysis syndrome (hyperuricemia, hyperphosphatemia, and hypocalcemia). He responded well to hydration and diuretics. However, after ALPPS stage-2, the tumor lysis syndrome, which was initially well-controlled, suddenly progressed into renal failure with concomitant respiratory dysfunction. Continuous renal replacement therapy (CRRT) and mechanical ventilation were administered to manage the multiple organ dysfunction (MOD), but he died three days later. PHLF was also common but required no special treatments in most patients (2 grade A PHLF after ALPPS stage-1; 1 grade A PHLF after TAE; 5 grade A PHLF and 2 grade B PHLF after ALPPS stage-2, respectively). Most patients presented with large amounts of peritoneal drainage after ALPPS stage-1/2 (Figure S2B). Four patients developed ascites after ALPPS stage-1 while 3 patients after ALPPS stage-2 (Table S1). Pleural effusion and bile leakage occurred in case-5 and case-9, respectively. The pleural effusion responded well to thoracocentesis. Anemia presented in 2 patients (case-5 and case-10) after ALPPS stage-2. Red blood transfusion was given to 1 patient (case-5), while erythropoietin was administered for 2 weeks to another patient (case-10). All the other patients recovered uneventfully.
Laboratory findings include total bilirubin (TB), international normalized ratio (INR), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and serum creatinine (SCr) levels after ALPPS stage-1, TAE and ALPPS stage-2 are shown in Figure S2C-S2H. Using the definition and grading system of the ISGLS, 2 patients (2/10, 20.0%) developed grade A PHLF after ALPPS stage-1, while 1 patient (1/10, 10.0%) developed grade A PHLF after TAE (Table S1). However, after ALPPS stage-2, 5 patients (5/10, 50.0%) developed grade A PHLF, and 2 patients (2/10, 20.0%) developed grade B PHLF (Table S1). PHLF was more commonly seen after ALPPS stage-2. All patients with PHLF recovered well with conservative managements which required no administration of special drugs. In 9/10 patients (90.0%), tumor lysis occurring after ligation of portal vein and embolization of supplying arteries to tumors was well controlled with no increase in uric acid (UA) and SCr to abnormally high levels. Only one patient (1/10, 10.0%) developed severe tumor lysis after ALPPS stage-1. The UA significantly elevated on POD1 to 444 µmol/L which gradually increased to reach 618 µmol/L on POD13. After TAE, it kept on increasing to reach 743 µmol/L on day 7 after TAE. The SCr also increased on POD2 to reach 237 µmol/L. It then returned to normal on POD9 after ALPPS stage-1. The SCr increased to 120 µmol/L after TAE to reach 179 µmol/L on day 5 after TAE. After ALPPS stage-2 when the tumor was removed, with CRRT, their levels decreased to normal.
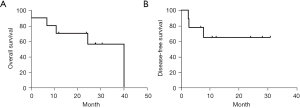
Negative resection margins (R0 resections) were confirmed by histological examination in all 10 patients (100%). Postoperative adjuvant TACE was given to 2 patients one month after ALPPS stage-2 and these patients recovered uneventfully. The median follow-up for all patients was 24.1 (range, 0.1–40.0) months. The median survival was 40 months (Figure 3). One patient developed MOD and died on POD3 after ALPPS stage-2. Three patients (case-1, case-3 and case-8) developed recurrences in the FLR. The disease-free survivals (DFS) of these three patients were 2.5, 2.7 and 7.8 months, respectively. Open resection of recurrent tumor was performed in 1 patient (case-1). He developed re-recurrence 7 months later and finally died of tumor progression at 24.4 months. TACE was performed in the remaining 2 patients (case-3 and case-8) who were alive and well at the time of censor of this study. All the other patients had no evidence of recurrence.
Discussion
The results of this study showed TAE-salvaged ALPPS to be especially suitable for patients with HCC and fibrosis/cirrhosis who had failed to induce adequate volumes of FLR after conventional ALPPS stage-1. R0 resection was achieved in all these patients (100.0%) in ALPPS stage-2 after TAE-salvaged ALPPS. The median survival was 40 months which is comparable to the survival of large tumors treated with one-stage resection (22,23).
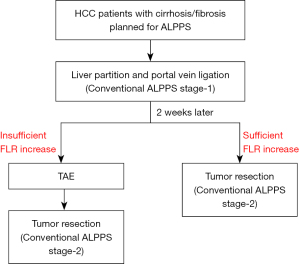
In our previous study, the median KGRs of FLR in patients with cirrhosis who underwent conventional ALPPS were 16.45 (range, 5.64–34.33) mL/day in the first week and 8.43 (range, 2.29–18.33) mL/day in the second week after ALPPS stage-1. Due to the limited FLR hypertrophy and the gradual attenuation of hypertrophy with time after ALPPS stage-1, patients with cirrhosis or severe fibrosis often have to endure a long wait for ALPPS stage-2, tumor progression, high morbidity and mortality, and subsequently increased risks of ALPPS failure as compared with patients with a normal liver. In our learning curve on ALPPS, 4 patients with cirrhosis failed to develop adequate hypertrophy of FLR volumes to complete ALPPS stage-2. Two of the 4 patients were converted to other treatments, while the other 2 patients present with irreversible PHLF and died unfortunately. The use of TAE-salvaged ALPPS on the 10 patients (6 with cirrhosis, 1 with S3-fibrosis, and 3 with S2-fibrosis) in this study all led to successful development of adequate FLR volumes to complete ALPPS stage-2. These patients would have otherwise to be considered as failure of ALPPS and converted to receive palliative treatments. Thus, TAE-salvaged ALPPS is suitable for patients with HCC and severe fibrosis/cirrhosis who are planned to undergo ALPPS but fail to develop adequate FLR hypertrophy after conventional ALPPS stage-1. A new algorithm of ALPPS for patients with HCC and fibrosis/cirrhosis is shown in Figure 4.
The mechanism underlying induction of significant hypertrophy of FLR by TAE-Salvage ALPPS is probably related to the amount of arterial supply to the FLR. Arterial blood supply is critical for liver regeneration. In severe cases of small-for-size grafts in liver transplantation, poor hepatic arterial flow leads to functional de-arterialization and parenchymal infarcts. Furthermore, partial hepatectomy accompanied by ligation of its hepatic arterial supply results in failure of the remnant liver to regenerate (24,25). In contrast with non-HCC tumor (such as CRLM), the HCC is hypervascular with their main blood supplies coming from hepatic arteries. And ALPPS for HCC is commonly carried out for huge tumors which often occupy large amount of arterial blood. The huge HCCs can “steal” arterial blood from the remnant liver, with aggravation of the ‘steal’ after ALPPS stage-1 (9). This can significantly reduce the arterial supply to FLR and limit the rate and degree of FLR hypertrophy. And it is postulated to be more severe in HCC patients as compared with non-HCC patients who had no fibrosis/cirrhosis because the fibrosis/cirrhosis has negative impact on FLR hypertrophy. Furthermore, the hepatic arterial buffer response (HABR) can also play an important role in regulating hepatic arterial blood flow to FLR (25). It has been observed that there is increased compensatory arterial flow after portal vein embolization or ligation, and the increased compensatory arterial flow can encourage tumor progression (26,27). Ligation of right portal vein has been shown to result in reduction of portal venous inflow to right liver, leading to HABR-induced right hepatic artery dilation (25). Nagino et al. (28) reported an immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization. Several studies also showed that when all portal venous blood flows through a small-for-size liver graft in living donor liver transplantation, the built-up pressure in the portal vein can effectively shut down the hepatic arterial flow, and the liver becomes “dearterialized” (24,25). Thus, both the arterial blood ‘steal’ by tumor and HABR caused by right portal vein ligation can lead to a decrease in arterial supply to FLR which eventually hamper the degree and rate of hypertrophy of FLR. Our results showed that the cross-sectional area ratios (left vs. right hepatic arteries) before operation, two weeks after ALPPS stage-1 and one week after TAE were 27.16% (range, 12.81–172.98%), 13.37% (range, 7.44–55.15%), and 22.41% (range, 13.22–50.36%), respectively (Figure S3). These results indicated that TAE of tumor significantly improved the arterial supply to the FLR, abrogated the arterial steal and significantly decreased the HABR-induced responses.
Conventional ALPPS, especially carried out on patients with HCC and fibrosis/cirrhosis, is associated with relatively high morbidity and mortality rates. Our initial experience showed that the new TAE-salvaged ALPPS did not present significantly higher morbidity and mortality rates when compared with conventional ALPPS. The morbidity rates of TAE-salvaged ALPPS were 50.0% (5/10), 20.0% (2/10) and 70.0% (7/10) after ALPPS stage-1, TAE and ALPPS stage-2, respectively. By contrast, the reported morbidity rates of conventional ALPPS were 37.8% (17/45) and 56.1% (23/41) after ALPPS stage-1 and ALPPS stage-2, respectively. The mortality rates after completion of the TAE-salvaged ALPPS was 10.0%, while that reported after conventional ALPPS in patients with HCC was 11.1–31% (3,8). The patient-4 in this study died unfortunately. He was with a huge tumor (diameter of 17 cm) and developed tumor lysis syndrome after ALPPS stage-1 and TAE. He responded well to hydration and diuretics. However, after ALPPS stage-2, the tumor lysis syndrome suddenly progressed into renal failure with concomitant acute respiratory failure. He finally died of the renal and respiratory complications. The tumor lysis and renal function after ALPPS stage-1 and TAE should be cautiously monitored and paid close attention to treatment.
TAE-salvaged ALPPS enabled a 100% R0 resection rate in patients with HCC and severe fibrosis/cirrhosis in this study. Before using this novel TAE-salvaged ALPPS, there were 16 patients with cirrhosis who underwent ALPPS in our hospital, and 4 patients failed to complete ALPPS stage-2 and the tumor resection rate was only 75% (12/16). The new TAE-salvaged ALPPS enabled the R0 resection rate of 100%, with a short interval to proceed to ALPPS stage-2 within 21 days. The median overall survival for these 10 patients (median diameter 14 cm) was acceptable 40 months.
There are limitations of this study. First, this is a retrospective study with its inherent defects. Second, the sample size is relatively small. Third, the study came from a tertiary referral center in China where the etiology of HCC is predominantly chronic hepatitis B viral infection. Whether the results of this study can be extrapolated to other populations with different etiologies of HCC require further studies.
In conclusion, the new TAE-salvaged ALPPS can induce fast FLR hypertrophy and control tumor progression in patients with HCC and fibrosis/cirrhosis. It does not significantly increase operative morbidity and mortality rates as compared with conventional ALPPS. It can serve as a new strategy to increase the resectability of HCC in patients with cirrhosis or fibrosis who failed to develop sufficient hypertrophy of FLR after conventional ALPPS stage-1. However, more prospective studies are needed to further evaluate the effectiveness, safety and long-term oncological outcomes of this new ALPPS procedure.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 82150004).
Footnote
Reporting Checklist: The authors have completed the STROCSS reporting checklist. Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-466/rc
Data Sharing Statement: Available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-466/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-21-466/coif). WYL serves as the unpaid advisor of Hepatobiliary Surgery and Nutrition. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Zhongshan Hospital Fudan University. (No. B2021-879) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lang H, de Santibañes E, Schlitt HJ, et al. 10th Anniversary of ALPPS-Lessons Learned and quo Vadis. Ann Surg 2019;269:114-9. [Crossref] [PubMed]
- Sandström P, Røsok BI, Sparrelid E, et al. ALPPS Improves Resectability Compared With Conventional Two-stage Hepatectomy in Patients With Advanced Colorectal Liver Metastasis: Results From a Scandinavian Multicenter Randomized Controlled Trial (LIGRO Trial). Ann Surg 2018;267:833-40. [Crossref] [PubMed]
- D'Haese JG, Neumann J, Weniger M, et al. Should ALPPS be Used for Liver Resection in Intermediate-Stage HCC? Ann Surg Oncol 2016;23:1335-43. [Crossref] [PubMed]
- van Lienden KP, van den Esschert JW, de Graaf W, et al. Portal vein embolization before liver resection: a systematic review. Cardiovasc Intervent Radiol 2013;36:25-34. [Crossref] [PubMed]
- Chan AC, Poon RT, Chan C, et al. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg 2016;263:e14-6. [Crossref] [PubMed]
- Vennarecci G, Grazi GL, Sperduti I, et al. ALPPS for primary and secondary liver tumors. Int J Surg 2016;30:38-44. [Crossref] [PubMed]
- Vennarecci G, Laurenzi A, Levi Sandri GB, et al. The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol 2014;40:982-8. [Crossref] [PubMed]
- Wang Z, Peng Y, Hu J, et al. Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy for Unresectable Hepatitis B Virus-related Hepatocellular Carcinoma: A Single Center Study of 45 Patients. Ann Surg 2020;271:534-41. [Crossref] [PubMed]
- Wang Z, Peng Y, Sun Q, et al. Salvage transhepatic arterial embolization after failed stage I ALPPS in a patient with a huge HCC with chronic liver disease: A case report. Int J Surg Case Rep 2017;39:131-5. [Crossref] [PubMed]
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-93. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Zhou J, Sun H, Wang Z, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer 2020;9:682-720. [Crossref] [PubMed]
- Zhuang Y, Ding H, Zhang Y, et al. Two-dimensional Shear-Wave Elastography Performance in the Noninvasive Evaluation of Liver Fibrosis in Patients with Chronic Hepatitis B: Comparison with Serum Fibrosis Indexes. Radiology 2017;283:873-82. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery 2011;149:713-24. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ishizawa T, Hasegawa K, Kokudo N, et al. Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg 2009;144:46-51. [Crossref] [PubMed]
- Rahbari NN, Garden OJ, Padbury R, et al. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS). HPB (Oxford) 2011;13:528-35. [Crossref] [PubMed]
- Koch M, Garden OJ, Padbury R, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery 2011;149:680-8. [Crossref] [PubMed]
- Yin X, Zhang L, Wang YH, et al. Transcatheter arterial chemoembolization combined with radiofrequency ablation delays tumor progression and prolongs overall survival in patients with intermediate (BCLC B) hepatocellular carcinoma. BMC Cancer 2014;14:849. [Crossref] [PubMed]
- Wang Z, Ren Z, Chen Y, et al. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res 2018;24:2074-81. [Crossref] [PubMed]
- Urata K, Hashikura Y, Ikegami T, et al. Standard liver volume in adults. Transplant Proc 2000;32:2093-4. [Crossref] [PubMed]
- Shrager B, Jibara GA, Tabrizian P, et al. Resection of large hepatocellular carcinoma (≥10 cm): a unique western perspective. J Surg Oncol 2013;107:111-7. [Crossref] [PubMed]
- Yang LY, Fang F, Ou DP, et al. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg 2009;249:118-23. [Crossref] [PubMed]
- Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 2010;176:2-13. [Crossref] [PubMed]
- Eipel C, Abshagen K, Vollmar B. Regulation of hepatic blood flow: the hepatic arterial buffer response revisited. World J Gastroenterol 2010;16:6046-57. [Crossref] [PubMed]
- Ribero D, Curley SA, Imamura H, et al. Selection for resection of hepatocellular carcinoma and surgical strategy: indications for resection, evaluation of liver function, portal vein embolization, and resection. Ann Surg Oncol 2008;15:986-92. [Crossref] [PubMed]
- Shindoh J, D, Tzeng CW, Vauthey JN. Portal vein embolization for hepatocellular carcinoma. Liver Cancer 2012;1:159-67. [Crossref] [PubMed]
- Nagino M, Nimura Y, Kamiya J, et al. Immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization: CT demonstration. AJR Am J Roentgenol 1998;171:1037-9. [Crossref] [PubMed]


